In Vitro Efficacy of Bacterial Cellulose Dressings Chemisorbed with Antiseptics against Biofilm Formed by Pathogens Isolated from Chronic Wounds
- PMID: 33924416
- PMCID: PMC8069587
- DOI: 10.3390/ijms22083996
In Vitro Efficacy of Bacterial Cellulose Dressings Chemisorbed with Antiseptics against Biofilm Formed by Pathogens Isolated from Chronic Wounds
Abstract
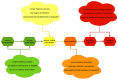
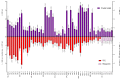
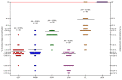
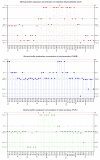
Local administration of antiseptics is required to prevent and fight against biofilm-based infections of chronic wounds. One of the methods used for delivering antiseptics to infected wounds is the application of dressings chemisorbed with antimicrobials. Dressings made of bacterial cellulose (BC) display several features, making them suitable for such a purpose. This work aimed to compare the activity of commonly used antiseptic molecules: octenidine, polyhexanide, povidone-iodine, chlorhexidine, ethacridine lactate, and hypochlorous solutions and to evaluate their usefulness as active substances of BC dressings against 48 bacterial strains (8 species) and 6 yeast strains (1 species). A silver dressing was applied as a control material of proven antimicrobial activity. The methodology applied included the assessment of minimal inhibitory concentrations (MIC) and minimal biofilm eradication concentration (MBEC), the modified disc-diffusion method, and the modified antibiofilm dressing activity measurement (A.D.A.M.) method. While in 96-well plate-based methods (MIC and MBEC assessment), the highest antimicrobial activity was recorded for chlorhexidine, in the modified disc-diffusion method and in the modified A.D.A.M test, povidone-iodine performed the best. In an in vitro setting simulating chronic wound conditions, BC dressings chemisorbed with polyhexanide, octenidine, or povidone-iodine displayed a similar or even higher antibiofilm activity than the control dressing containing silver molecules. If translated into clinical conditions, the obtained results suggest high applicability of BC dressings chemisorbed with antiseptics to eradicate biofilm from chronic wounds.
Keywords: antiseptics; bacterial cellulose; chronic wounds; dressing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





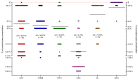

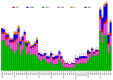

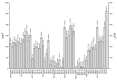



Similar articles
-
Delivery of antiseptic solutions by a bacterial cellulose wound dressing: Uptake, release and antibacterial efficacy of octenidine and povidone-iodine.Burns. 2020 Jun;46(4):918-927. doi: 10.1016/j.burns.2019.10.006. Epub 2019 Oct 23. Burns. 2020. PMID: 31653329
-
The cross-linked bacterial cellulose impregnated with octenidine dihydrochloride-based antiseptic as an antibacterial dressing material for highly-exuding, infected wounds.Microbiol Res. 2022 Oct;263:127125. doi: 10.1016/j.micres.2022.127125. Epub 2022 Jul 15. Microbiol Res. 2022. PMID: 35878492
-
An in vitro model of chronic wound biofilms to test wound dressings and assess antimicrobial susceptibilities.J Antimicrob Chemother. 2010 Jun;65(6):1195-206. doi: 10.1093/jac/dkq105. Epub 2010 Apr 8. J Antimicrob Chemother. 2010. PMID: 20378671
-
Update on the role of antiseptics in the management of chronic wounds with critical colonisation and/or biofilm.Int Wound J. 2021 Jun;18(3):342-358. doi: 10.1111/iwj.13537. Epub 2020 Dec 13. Int Wound J. 2021. PMID: 33314723 Free PMC article. Review.
-
Addressing the challenges in antisepsis: focus on povidone iodine.Int J Antimicrob Agents. 2020 Sep;56(3):106064. doi: 10.1016/j.ijantimicag.2020.106064. Epub 2020 Jun 26. Int J Antimicrob Agents. 2020. PMID: 32599228 Review.
Cited by
-
Antimicrobial and Cytotoxic Activities of Water-Soluble Isoxazole-Linked 1,3,4-Oxadiazole with Delocalized Charge: In Vitro and In Vivo Results.Int J Mol Sci. 2023 Nov 7;24(22):16033. doi: 10.3390/ijms242216033. Int J Mol Sci. 2023. PMID: 38003222 Free PMC article.
-
Culture Shock: An Investigation into the Tolerance of Pathogenic Biofilms to Antiseptics in Environments Resembling the Chronic Wound Milieu.Int J Mol Sci. 2023 Dec 8;24(24):17242. doi: 10.3390/ijms242417242. Int J Mol Sci. 2023. PMID: 38139071 Free PMC article.
-
Microbial Biofilms and Antibiofilm Agents 2.0.Int J Mol Sci. 2022 Jul 19;23(14):7932. doi: 10.3390/ijms23147932. Int J Mol Sci. 2022. PMID: 35887278 Free PMC article.
-
Assessment of Bacterial Nanocellulose Loaded with Acetylsalicylic Acid or Povidone-Iodine as Bioactive Dressings for Skin and Soft Tissue Infections.Pharmaceutics. 2022 Aug 9;14(8):1661. doi: 10.3390/pharmaceutics14081661. Pharmaceutics. 2022. PMID: 36015286 Free PMC article.
-
A New Source of Diterpene Lactones From Andrographis paniculata (Burm. f.) Nees-Two Endophytic Fungi of Colletotrichum sp. With Antibacterial and Antioxidant Activities.Front Microbiol. 2022 Feb 28;13:819770. doi: 10.3389/fmicb.2022.819770. eCollection 2022. Front Microbiol. 2022. PMID: 35295309 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

