Bacillus velezensis CE 100 Inhibits Root Rot Diseases (Phytophthora spp.) and Promotes Growth of Japanese Cypress (Chamaecyparis obtusa Endlicher) Seedlings
- PMID: 33924463
- PMCID: PMC8069221
- DOI: 10.3390/microorganisms9040821
Bacillus velezensis CE 100 Inhibits Root Rot Diseases (Phytophthora spp.) and Promotes Growth of Japanese Cypress (Chamaecyparis obtusa Endlicher) Seedlings
Abstract
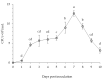
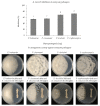
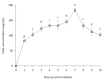
Root rot diseases, caused by phytopathogenic oomycetes, Phytophthora spp. cause devastating losses involving forest seedlings, such as Japanese cypress (Chamaecyparis obtusa Endlicher) in Korea. Plant growth-promoting rhizobacteria (PGPR) are a promising strategy to control root rot diseases and promote growth in seedlings. In this study, the potential of Bacillus velezensis CE 100 in controlling Phytophthora root rot diseases and promoting the growth of C. obtusa seedlings was investigated. B. velezensis CE 100 produced β-1,3-glucanase and protease enzymes, which degrade the β-glucan and protein components of phytopathogenic oomycetes cell-wall, causing mycelial growth inhibition of P. boehmeriae, P. cinnamomi, P. drechsleri and P. erythoroseptica by 54.6%, 62.6%, 74.3%, and 73.7%, respectively. The inhibited phytopathogens showed abnormal growth characterized by swelling and deformation of hyphae. B. velezensis CE 100 increased the survival rate of C. obtusa seedlings 2.0-fold and 1.7-fold compared to control, and fertilizer treatment, respectively. Moreover, B. velezensis CE 100 produced indole-3-acetic acid (IAA) up to 183.7 mg/L, resulting in a significant increase in the growth of C. obtusa seedlings compared to control, or chemical fertilizer treatment, respectively. Therefore, this study demonstrates that B. velezensis CE 100 could simultaneously control Phytophthora root rot diseases and enhance growth of C. obtusa seedlings.
Keywords: antagonistic bacteria; auxin; biocontrol agent; forest seedling production; lytic enzymes; phytopathogenic oomycetes; plant development.
Conflict of interest statement
The authors have no conflicts of interest relevant to this study to disclose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Matsumoto A., Tani N., LI X.G., Nakao Y., Tomaru N., Tsumura Y. Development and polymorphisms of microsatellite markers for hinoki (Chamaecyparis obtusa) Mol. Ecol. Resour. 2006;6:310–312. doi: 10.1111/j.1471-8286.2006.01212.x. - DOI
-
- Tian M., Han D., Row K.H. Preparation of molecularly imprinted polymer for extracting flavones from Chamaecyparis obtusa. Anal. Lett. 2011;44:737–746. doi: 10.1080/00032711003783176. - DOI
-
- Tang B., Bi W., Zhang H., Row K.H. Deep eutectic solvent-based HS-SME coupled with GC for the analysis of bioactive terpenoids in Chamaecyparis obtusa leaves. Chromatographia. 2014;77:373–377. doi: 10.1007/s10337-013-2607-3. - DOI
-
- Shik M.H., Woon K.T., Solomon T. Growth Performance Assessment of Chamaecyparis obtusa Stand in Gyeongnam Province, S. Korea. Agric. For. Fish. 2020;9:135–141. doi: 10.11648/j.aff.20200905.11. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

