Evaluating Oxygen Tensions Related to Bone Marrow and Matrix for MSC Differentiation in 2D and 3D Biomimetic Lamellar Scaffolds
- PMID: 33924614
- PMCID: PMC8068918
- DOI: 10.3390/ijms22084010
Evaluating Oxygen Tensions Related to Bone Marrow and Matrix for MSC Differentiation in 2D and 3D Biomimetic Lamellar Scaffolds
Abstract
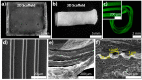
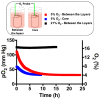
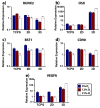
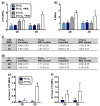
The physiological O2 microenvironment of mesenchymal stem cells (MSCs) and osteoblasts and the dimensionality of a substrate are known to be important in regulating cell phenotype and function. By providing the physiologically normoxic environments of bone marrow (5%) and matrix (12%), we assessed their potential to maintain stemness, induce osteogenic differentiation, and enhance the material properties in the micropatterned collagen/silk fibroin scaffolds that were produced in 2D or 3D. Expression of osterix (OSX) and vascular endothelial growth factor A (VEGFA) was significantly enhanced in the 3D scaffold in all oxygen environments. At 21% O2, OSX and VEGFA expressions in the 3D scaffold were respectively 13,200 and 270 times higher than those of the 2D scaffold. Markers for assessing stemness were significantly more pronounced on tissue culture polystyrene and 2D scaffold incubated at 5% O2. At 21% O2, we measured significant increases in ultimate tensile strength (p < 0.0001) and Young's modulus (p = 0.003) of the 3D scaffold compared to the 2D scaffold, whilst 5% O2 hindered the positive effect of cell seeding on tensile strength. In conclusion, we demonstrated that the 3D culture of MSCs in collagen/silk fibroin scaffolds provided biomimetic cues for bone progenitor cells toward differentiation and enhanced the tensile mechanical properties.
Keywords: 2D vs. 3D; bone tissue engineering; mesenchymal stem cell; osteogenesis; oxygen tension.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Matrix dimensionality and stiffness cooperatively regulate osteogenesis of mesenchymal stromal cells.Acta Biomater. 2016 Mar 1;32:210-222. doi: 10.1016/j.actbio.2016.01.010. Epub 2016 Jan 11. Acta Biomater. 2016. PMID: 26790775
-
Chondrogenic differentiation of rat MSCs on porous scaffolds of silk fibroin/chitosan blends.Biomaterials. 2012 Apr;33(10):2848-57. doi: 10.1016/j.biomaterials.2011.12.028. Epub 2012 Jan 17. Biomaterials. 2012. PMID: 22261099
-
Stromal-cell-derived extracellular matrix promotes the proliferation and retains the osteogenic differentiation capacity of mesenchymal stem cells on three-dimensional scaffolds.Tissue Eng Part C Methods. 2015 Feb;21(2):171-81. doi: 10.1089/ten.TEC.2014.0092. Epub 2014 Aug 4. Tissue Eng Part C Methods. 2015. PMID: 24965227 Free PMC article.
-
Co-culture cell-derived extracellular matrix loaded electrospun microfibrous scaffolds for bone tissue engineering.Mater Sci Eng C Mater Biol Appl. 2019 Jun;99:479-490. doi: 10.1016/j.msec.2019.01.127. Epub 2019 Jan 30. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30889723 Free PMC article.
-
Strain and Vibration in Mesenchymal Stem Cells.Int J Biomater. 2018 Jan 9;2018:8686794. doi: 10.1155/2018/8686794. eCollection 2018. Int J Biomater. 2018. PMID: 29545825 Free PMC article. Review.
Cited by
-
Functionalized 3D scaffolds for engineering the hematopoietic niche.Front Bioeng Biotechnol. 2022 Aug 17;10:968086. doi: 10.3389/fbioe.2022.968086. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36061428 Free PMC article. Review.
-
Endothelin-1 receptor blockade impairs invasion patterns in engineered 3D high-grade serous ovarian cancer tumouroids.Clin Sci (Lond). 2024 Nov 20;138(22):1441-1450. doi: 10.1042/CS20240371. Clin Sci (Lond). 2024. PMID: 39503511 Free PMC article.
-
Harnessing the potential of oxygen-generating materials and their utilization in organ-specific delivery of oxygen.Biomater Sci. 2023 Feb 28;11(5):1567-1588. doi: 10.1039/d2bm01329k. Biomater Sci. 2023. PMID: 36688522 Free PMC article. Review.
-
Integrating physicomechanical and biological strategies for BTE: biomaterials-induced osteogenic differentiation of MSCs.Theranostics. 2023 May 21;13(10):3245-3275. doi: 10.7150/thno.84759. eCollection 2023. Theranostics. 2023. PMID: 37351163 Free PMC article. Review.
-
Mesenchymal stem cells from biology to therapy.Emerg Top Life Sci. 2021 Oct 29;5(4):539-548. doi: 10.1042/ETLS20200303. Emerg Top Life Sci. 2021. PMID: 34355761 Free PMC article.
References
-
- Baradaran T., Shafiei S.S., Mohammadi S., Moztarzadeh F. Poly (ε-caprolactone)/layered double hydroxide microspheres-aggregated nanocomposite scaffold for osteogenic differentiation of mesenchymal stem cell. Mater. Today Commun. 2020;23:100913. doi: 10.1016/j.mtcomm.2020.100913. - DOI
-
- Carlström I.E., Rashad A., Campodoni E., Sandri M., Syverud K., Bolstad A.I., Mustafa K. Cross-linked gelatin-nanocellulose scaffolds for bone tissue engineering. Mater. Lett. 2020;264:127326. doi: 10.1016/j.matlet.2020.127326. - DOI
-
- Silva J.C., Carvalho M.S., Udangawa R.N., Moura C.S., Cabral J.M.S., Da Silva C.L., Ferreira F.C., Vashishth D., Linhardt R.J. Extracellular matrix decorated polycaprolactone scaffolds for improved mesenchymal stem/stromal cell osteogenesis towards a patient-tailored bone tissue engineering approach. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020;108:2153–2166. doi: 10.1002/jbm.b.34554. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

