Complement Inactivation Strategy of Staphylococcus aureus Using Decay-Accelerating Factor and the Response of Infected HaCaT Cells
- PMID: 33924622
- PMCID: PMC8070078
- DOI: 10.3390/ijms22084015
Complement Inactivation Strategy of Staphylococcus aureus Using Decay-Accelerating Factor and the Response of Infected HaCaT Cells
Abstract
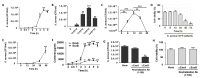
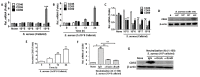
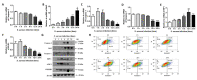
Staphylococcus aureus is a species of Gram-positive staphylococcus. It can cause sinusitis, respiratory infections, skin infections, and food poisoning. Recently, it was discovered that S. aureus infects epithelial cells, but the interaction between S. aureus and the host is not well known. In this study, we confirmed S. aureus to be internalized by HaCaT cells using the ESAT-6-like protein EsxB and amplified within the host over time by escaping host immunity. S. aureus increases the expression of decay-accelerating factor (CD55) on the surfaces of host cells, which inhibits the activation of the complement system. This mechanism makes it possible for S. aureus to survive in host cells. S. aureus, sufficiently amplified within the host, is released through the initiation of cell death. On the other hand, the infected host cells increase their surface expression of UL16 binding protein 1 to inform immune cells that they are infected and try to be eliminated. These host defense systems seem to involve the alteration of tight junctions and the induction of ligand expression to activate immune cells. Taken together, our study elucidates a novel aspect of the mechanisms of infection and immune system evasion for S. aureus.
Keywords: ESAT-6-like protein EsxB; Staphylococcus aureus; UL16 binding protein 1; decay-accelerating factor; internalization; tight junction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Deciphering Staphylococcus aureus-host dynamics using dual activity-based protein profiling of ATP-interacting proteins.mSystems. 2024 May 16;9(5):e0017924. doi: 10.1128/msystems.00179-24. Epub 2024 Apr 24. mSystems. 2024. PMID: 38656122 Free PMC article.
-
Combination treatment with lipoteichoic acids isolated from Lactobacillus plantarum and Staphylococcus aureus alleviates atopic dermatitis via upregulation of CD55 and CD59.Immunol Lett. 2019 Oct;214:23-29. doi: 10.1016/j.imlet.2019.08.005. Epub 2019 Aug 24. Immunol Lett. 2019. PMID: 31454521
-
Type VII secretion system extracellular protein B targets STING to evade host anti-Staphylococcus aureus immunity.Proc Natl Acad Sci U S A. 2024 May 28;121(22):e2402764121. doi: 10.1073/pnas.2402764121. Epub 2024 May 21. Proc Natl Acad Sci U S A. 2024. PMID: 38771879 Free PMC article.
-
The staphylococcal surface-glycopolymer wall teichoic acid (WTA) is crucial for complement activation and immunological defense against Staphylococcus aureus infection.Immunobiology. 2016 Oct;221(10):1091-101. doi: 10.1016/j.imbio.2016.06.003. Epub 2016 Jun 15. Immunobiology. 2016. PMID: 27424796 Review.
-
Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus.Front Cell Infect Microbiol. 2017 Jun 30;7:286. doi: 10.3389/fcimb.2017.00286. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28713774 Free PMC article. Review.
Cited by
-
Genomic epidemiology and phenotypic characterization of Staphylococcus aureus isolated from atopic dermatitis patients in South China.Sci Rep. 2025 Feb 8;15(1):4773. doi: 10.1038/s41598-025-87317-9. Sci Rep. 2025. PMID: 39922832 Free PMC article.
-
Staphylococcal trafficking and infection-from 'nose to gut' and back.FEMS Microbiol Rev. 2022 Jan 18;46(1):fuab041. doi: 10.1093/femsre/fuab041. FEMS Microbiol Rev. 2022. PMID: 34259843 Free PMC article. Review.
-
The influence of marine fungal meroterpenoid meroantarctine A toward HaCaT keratinocytes infected with Staphylococcus aureus.J Antibiot (Tokyo). 2024 Dec;77(12):812-822. doi: 10.1038/s41429-024-00771-x. Epub 2024 Sep 10. J Antibiot (Tokyo). 2024. PMID: 39256545
-
Staphylococcus aureus Infection: Pathogenesis and Antimicrobial Resistance.Int J Mol Sci. 2023 May 3;24(9):8182. doi: 10.3390/ijms24098182. Int J Mol Sci. 2023. PMID: 37175886 Free PMC article.
-
Staphylococcus aureus can use an alternative pathway to be internalized by osteoblasts in absence of β1 integrins.Sci Rep. 2024 Nov 19;14(1):28643. doi: 10.1038/s41598-024-78754-z. Sci Rep. 2024. PMID: 39562631 Free PMC article.
References
-
- Conrad W.H., Osman M.M., Shanahan J.K., Chu F., Takaki K.K., Cameron J., Hopkinson-Woolley D., Brosch R., Ramakrishnan L. Mycobacterial ESX-1 secretion system mediates host cell lysis through bacterium contact-dependent gross membrane disruptions. Proc. Natl. Acad. Sci. USA. 2017;114:1371–1376. doi: 10.1073/pnas.1620133114. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

