Cyan-Emitting Cu(I) Complexes and Their Luminescent Metallopolymers
- PMID: 33924921
- PMCID: PMC8125312
- DOI: 10.3390/molecules26092567
Cyan-Emitting Cu(I) Complexes and Their Luminescent Metallopolymers
Abstract
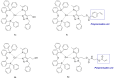
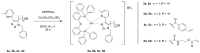
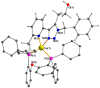
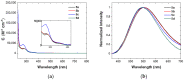
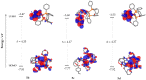
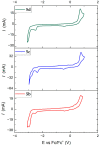
Copper complexes have shown great versatility and a wide application range across the natural and life sciences, with a particular promise as organic light-emitting diodes. In this work, four novel heteroleptic Cu(I) complexes were designed in order to allow their integration in advanced materials such as metallopolymers. We herein present the synthesis and the electrochemical and photophysical characterisation of these Cu(I) complexes, in combination with ab initio calculations. The complexes present a bright cyan emission (λem ~ 505 nm) in their solid state, both as powder and as blends in a polymer matrix. The successful synthesis of metallopolymers embedding two of the novel complexes is shown. These copolymers were also found to be luminescent and their photophysical properties were compared to those of their polymer blends. The chemical nature of the polymer backbone contributes significantly to the photoluminescence quantum yield, paving a route for the strategic design of novel luminescent Cu(I)-based polymeric materials.
Keywords: RuAAC; functional copolymers; heteroleptic copper(I) complexes; light-emitting metallopolymers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
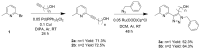
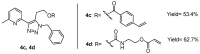

Similar articles
-
Wide-Bite-Angle Diphosphine Ligands in Thermally Activated Delayed Fluorescent Copper(I) Complexes: Impact on the Performance of Electroluminescence Applications.Inorg Chem. 2021 Jul 19;60(14):10323-10339. doi: 10.1021/acs.inorgchem.1c00804. Epub 2021 Jul 1. Inorg Chem. 2021. PMID: 34197094
-
New tetrazole-based Cu(I) homo- and heteroleptic complexes with various P^P ligands: synthesis, characterization, redox and photophysical properties.Dalton Trans. 2013 Jan 28;42(4):997-1010. doi: 10.1039/c2dt32056h. Dalton Trans. 2013. PMID: 23108182
-
Designing NHC-Copper(I) Dipyridylamine Complexes for Blue Light-Emitting Electrochemical Cells.ACS Appl Mater Interfaces. 2016 Jun 15;8(23):14678-91. doi: 10.1021/acsami.6b04647. Epub 2016 Jun 6. ACS Appl Mater Interfaces. 2016. PMID: 27224961
-
TADF Material Design: Photophysical Background and Case Studies Focusing on CuI and AgI Complexes.Chemphyschem. 2017 Dec 15;18(24):3508-3535. doi: 10.1002/cphc.201700872. Epub 2017 Dec 19. Chemphyschem. 2017. PMID: 29083512 Review.
-
Luminescent ionic transition-metal complexes for light-emitting electrochemical cells.Angew Chem Int Ed Engl. 2012 Aug 13;51(33):8178-211. doi: 10.1002/anie.201201471. Angew Chem Int Ed Engl. 2012. PMID: 22887710 Review.
Cited by
-
Metalation of a Hierarchical Self-Assembly Consisting of π-Stacked Cubes through Single-Crystal-to-Single-Crystal Transformation.Molecules. 2023 Jun 22;28(13):4923. doi: 10.3390/molecules28134923. Molecules. 2023. PMID: 37446584 Free PMC article.
-
Time-Resolved Spectroscopy and Electronic Structure of Mono-and Dinuclear Pyridyl-Triazole/DPEPhos-Based Cu(I) Complexes.Chemistry. 2021 Nov 2;27(61):15251-15270. doi: 10.1002/chem.202102760. Epub 2021 Oct 14. Chemistry. 2021. PMID: 34550622 Free PMC article.
-
A Red-Emitting Cu(I)-Halide Cluster Phosphor with Near-Unity Photoluminescence Efficiency for High-Power wLED Applications.Molecules. 2022 Jul 11;27(14):4441. doi: 10.3390/molecules27144441. Molecules. 2022. PMID: 35889315 Free PMC article.
References
-
- Demas J.N., DeGraff B.A. Applications of luminescent transition platinum group metal complexes to sensor technology and molecular probes. Coord. Chem. Rev. 2001;211:317–351. doi: 10.1016/S0010-8545(00)00278-2. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

