Molecular Targets of Manganese-Induced Neurotoxicity: A Five-Year Update
- PMID: 33925013
- PMCID: PMC8124173
- DOI: 10.3390/ijms22094646
Molecular Targets of Manganese-Induced Neurotoxicity: A Five-Year Update
Abstract
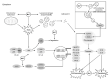
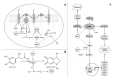
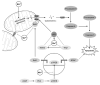
Understanding of the immediate mechanisms of Mn-induced neurotoxicity is rapidly evolving. We seek to provide a summary of recent findings in the field, with an emphasis to clarify existing gaps and future research directions. We provide, here, a brief review of pertinent discoveries related to Mn-induced neurotoxicity research from the last five years. Significant progress was achieved in understanding the role of Mn transporters, such as SLC39A14, SLC39A8, and SLC30A10, in the regulation of systemic and brain manganese handling. Genetic analysis identified multiple metabolic pathways that could be considered as Mn neurotoxicity targets, including oxidative stress, endoplasmic reticulum stress, apoptosis, neuroinflammation, cell signaling pathways, and interference with neurotransmitter metabolism, to name a few. Recent findings have also demonstrated the impact of Mn exposure on transcriptional regulation of these pathways. There is a significant role of autophagy as a protective mechanism against cytotoxic Mn neurotoxicity, yet also a role for Mn to induce autophagic flux itself and autophagic dysfunction under conditions of decreased Mn bioavailability. This ambivalent role may be at the crossroad of mitochondrial dysfunction, endoplasmic reticulum stress, and apoptosis. Yet very recent evidence suggests Mn can have toxic impacts below the no observed adverse effect of Mn-induced mitochondrial dysfunction. The impact of Mn exposure on supramolecular complexes SNARE and NLRP3 inflammasome greatly contributes to Mn-induced synaptic dysfunction and neuroinflammation, respectively. The aforementioned effects might be at least partially mediated by the impact of Mn on α-synuclein accumulation. In addition to Mn-induced synaptic dysfunction, impaired neurotransmission is shown to be mediated by the effects of Mn on neurotransmitter systems and their complex interplay. Although multiple novel mechanisms have been highlighted, additional studies are required to identify the critical targets of Mn-induced neurotoxicity.
Keywords: apoptosis; cell signaling; manganese; neuroinflammation; neurotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Martinez-Finley E.J., Chakraborty S., Aschner M. In: Manganese in Biological Systems. Encyclopedia of Metalloproteins. Kretsinger R.H., Uversky V.N., Permyakov E.A., editors. Springer; New York, NY, USA: 2013. pp. 1297–1303.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

