AAV-HDV: An Attractive Platform for the In Vivo Study of HDV Biology and the Mechanism of Disease Pathogenesis
- PMID: 33925087
- PMCID: PMC8145145
- DOI: 10.3390/v13050788
AAV-HDV: An Attractive Platform for the In Vivo Study of HDV Biology and the Mechanism of Disease Pathogenesis
Abstract
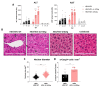
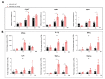
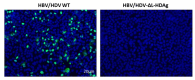
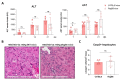
Hepatitis delta virus (HDV) infection causes the most severe form of viral hepatitis, but little is known about the molecular mechanisms involved. We have recently developed an HDV mouse model based on the delivery of HDV replication-competent genomes using adeno-associated vectors (AAV), which developed a liver pathology very similar to the human disease and allowed us to perform mechanistic studies. We have generated different AAV-HDV mutants to eliminate the expression of HDV antigens (HDAgs), and we have characterized them both in vitro and in vivo. We confirmed that S-HDAg is essential for HDV replication and cannot be replaced by L-HDAg or host cellular proteins, and that L-HDAg is essential to produce the HDV infectious particle and inhibits its replication. We have also found that lack of L-HDAg resulted in the increase of S-HDAg expression levels and the exacerbation of liver damage, which was associated with an increment in liver inflammation but did not require T cells. Interestingly, early expression of L-HDAg significantly ameliorated the liver damage induced by the mutant expressing only S-HDAg. In summary, the use of AAV-HDV represents a very attractive platform to interrogate in vivo the role of viral components in the HDV life cycle and to better understand the mechanism of HDV-induced liver pathology.
Keywords: AAV; HDAg; HDV; liver damage; mouse model.
Conflict of interest statement
The authors declare no conflict of interest in relation with the work presented in this manuscript.
Figures


Similar articles
-
Deciphering the Role of Post-Translational Modifications and Cellular Location of Hepatitis Delta Virus (HDV) Antigens in HDV-Mediated Liver Damage in Mice.Viruses. 2024 Feb 28;16(3):379. doi: 10.3390/v16030379. Viruses. 2024. PMID: 38543745 Free PMC article.
-
A new HDV mouse model identifies mitochondrial antiviral signaling protein (MAVS) as a key player in IFN-β induction.J Hepatol. 2017 Oct;67(4):669-679. doi: 10.1016/j.jhep.2017.05.010. Epub 2017 May 18. J Hepatol. 2017. PMID: 28527664
-
Casein kinase II and protein kinase C modulate hepatitis delta virus RNA replication but not empty viral particle assembly.J Virol. 1996 Sep;70(9):6190-8. doi: 10.1128/JVI.70.9.6190-6198.1996. J Virol. 1996. PMID: 8709245 Free PMC article.
-
Functional and clinical significance of hepatitis D virus genotype II infection.Curr Top Microbiol Immunol. 2006;307:173-86. doi: 10.1007/3-540-29802-9_9. Curr Top Microbiol Immunol. 2006. PMID: 16903226 Review.
-
[Hepatitis delta virus replication and the role of the small hepatitis delta protein S-HDAg].Med Sci (Paris). 2018 Oct;34(10):833-841. doi: 10.1051/medsci/2018209. Epub 2018 Nov 19. Med Sci (Paris). 2018. PMID: 30451678 Review. French.
Cited by
-
Hepatitis Delta Virus and Hepatocellular Carcinoma.Pathogens. 2024 Apr 27;13(5):362. doi: 10.3390/pathogens13050362. Pathogens. 2024. PMID: 38787214 Free PMC article. Review.
-
Deciphering the Role of Post-Translational Modifications and Cellular Location of Hepatitis Delta Virus (HDV) Antigens in HDV-Mediated Liver Damage in Mice.Viruses. 2024 Feb 28;16(3):379. doi: 10.3390/v16030379. Viruses. 2024. PMID: 38543745 Free PMC article.
-
Hepatitis B-Induced Hepatocellular Carcinoma: Understanding Viral Carcinogenesis and Disease Management.J Clin Med. 2025 Apr 7;14(7):2505. doi: 10.3390/jcm14072505. J Clin Med. 2025. PMID: 40217955 Free PMC article. Review.
-
Topoisomerase Inhibitors Increase Episomal DNA Expression by Inducing the Integration of Episomal DNA in Hepatic Cells.Pharmaceutics. 2023 Oct 13;15(10):2459. doi: 10.3390/pharmaceutics15102459. Pharmaceutics. 2023. PMID: 37896219 Free PMC article.
-
Adaptive Immune Responses, Immune Escape and Immune-Mediated Pathogenesis during HDV Infection.Viruses. 2022 Jan 20;14(2):198. doi: 10.3390/v14020198. Viruses. 2022. PMID: 35215790 Free PMC article. Review.
References
-
- Kamal H., Westman G., Falconer K., Duberg A.S., Weiland O., Haverinen S., Wejstål R., Carlsson T., Kampmann C., Larsson S.B., et al. Long-Term Study of Hepatitis Delta Virus Infection at Secondary Care Centers: The Impact of Viremia on Liver-Related Outcomes. Hepatology. 2020;72:1177–1190. doi: 10.1002/hep.31214. - DOI - PubMed
-
- Palom A., Rodríguez-Tajes S., Navascués C.A., García-Samaniego J., Riveiro-Barciela M., Lens S., Lens S., Rodríguez M., Esteban R., Buti M. Long-term clinical outcomes in patients with chronic hepatitis delta: The role of persistent viraemia. Aliment. Pharm. Ther. 2020;51:158–166. doi: 10.1111/apt.15521. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

