Broadly Antiviral Activities of TAP1 through Activating the TBK1-IRF3-Mediated Type I Interferon Production
- PMID: 33925089
- PMCID: PMC8125511
- DOI: 10.3390/ijms22094668
Broadly Antiviral Activities of TAP1 through Activating the TBK1-IRF3-Mediated Type I Interferon Production
Abstract
Deeply understanding the virus-host interaction is a prerequisite for developing effective anti-viral strategies. Traditionally, the transporter associated with antigen processing type 1 (TAP1) is critical for antigen presentation to regulate adaptive immunity. However, its role in controlling viral infections through modulating innate immune signaling is not yet fully understood. In the present study, we reported that TAP1, as a product of interferon-stimulated genes (ISGs), had broadly antiviral activity against various viruses such as herpes simplex virus 1 (HSV-1), adenoviruses (AdV), vesicular stomatitis virus (VSV), dengue virus (DENV), Zika virus (ZIKV), and influenza virus (PR8) etc. This antiviral activity by TAP1 was further confirmed by series of loss-of-function and gain-of-function experiments. Our further investigation revealed that TAP1 significantly promoted the interferon (IFN)-β production through activating the TANK binding kinase-1 (TBK1) and the interferon regulatory factor 3 (IRF3) signaling transduction. Our work highlighted the broadly anti-viral function of TAP1 by modulating innate immunity, which is independent of its well-known function of antigen presentation. This study will provide insights into developing novel vaccination and immunotherapy strategies against emerging infectious diseases.
Keywords: broadly antiviral activity; innate immunity; interferon-stimulated genes (ISGs); transporter associated with antigen processing 1 (TAP1); type I interferons (IFN-Is).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

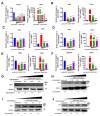
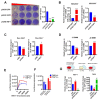
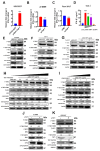

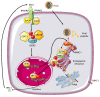
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

