Radiation for Oligometastatic Lung Cancer in the Era of Immunotherapy: What Do We (Need to) Know?
- PMID: 33925139
- PMCID: PMC8125691
- DOI: 10.3390/cancers13092132
Radiation for Oligometastatic Lung Cancer in the Era of Immunotherapy: What Do We (Need to) Know?
Abstract
Oligometastatic cancer is recognized as a separate entity within the spectrum of metastatic disease. It was suggested that patients with oligometastatic disease can obtain long-term survival by giving local ablative therapy (LAT) to all visible disease locations. However, the true extent from which metastatic cancer should be called "oligometastatic" is unknown, although a consensus definition for oligometastatic disease is proposed by research organizations, such as the EORTC (maximum of five metastases in three organs). Different states of the oligometastatic disease are defined, such as synchronous vs. metachronous, oligopersistent vs. oligoprogressive disease. All clinical trials including patients with non-small cell lung cancer (NSCLC) are small and most are not randomized. Two small randomized phase II trials on synchronous disease showed an improvement in progression free survival, with the addition of LAT, and one also demonstrated an overall survival benefit. Immune checkpoint inhibitors (ICI) were not part of the treatment in these trials, while ICI significantly improved long-term outcomes of patients with metastatic NSCLC. Radiotherapy might improve the prognosis of patients treated with ICI because of its immunostimulatory effects and the possibility to eradicate metastatic deposits. Here, we summarize the data for adding ablative radiotherapy to the treatment of oligometastatic NSCLC, especially in the ICI era, and discuss the challenges of combined treatment.
Keywords: immune checkpoint inhibitor; immunotherapy; non-small cell lung cancer; oligometastatic; radiotherapy.
Conflict of interest statement
Stephanie Peeters declares no conflict of interest. Evert van Limbergen declares no conflict of interest. Lizza Hendriks declares no conflict of interest related to the current manuscript; (outside of current manuscript: research funding Roche Genentech, Boehringer Ingelheim, AstraZeneca (all institution); advisory board: Boehringer, BMS, Eli Lilly, Roche Genentech, Pfizer, Takeda, MSD, Boehringer Ingelheim, Amgen, AstraZeneca (all institution); speaker–MSD (institution); travel/conference reimbursement: Roche Genentech (self); mentorship program with key opinion leaders–funded by AstraZeneca; fees for educational webinars–Quadia (self); interview sessions funded by Roche Genentech (institution); local PI of clinical trials–AstraZeneca, Novartis, BMS, MSD/Merck, GSK, Takeda, Blueprint Medicines, Roche Genentech, Janssen Pharmaceuticals, Mirati). Dirk De Ruysscher declares no conflict of interest related to the current manuscript.
Figures
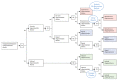
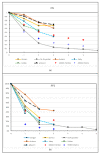
References
-
- Mok T., Camidge D.R., Gadgeel S.M., Rosell R., Dziadziuszko R., Kim D.W., Pérol M., Ou S.I., Ahn J.S., Shaw A.T., et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann. Oncol. 2020;31:1056–1064. doi: 10.1016/j.annonc.2020.04.478. - DOI - PubMed
-
- Brahmer J.R., Rodriguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. LBA51—KEYNOTE-024 5-year OS update: First-line (1L) pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) in patients (pts) with metastatic NSCLC and PD-L1 tumour proportion score (TPS) ≥50% Ann. Oncol. 2020;31:S1142–S1215. doi: 10.1016/j.annonc.2020.08.2284. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

