The Neuroprotective Effects of GPR4 Inhibition through the Attenuation of Caspase Mediated Apoptotic Cell Death in an MPTP Induced Mouse Model of Parkinson's Disease
- PMID: 33925146
- PMCID: PMC8125349
- DOI: 10.3390/ijms22094674
The Neuroprotective Effects of GPR4 Inhibition through the Attenuation of Caspase Mediated Apoptotic Cell Death in an MPTP Induced Mouse Model of Parkinson's Disease
Abstract
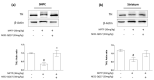
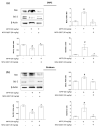
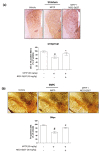
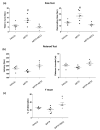
The proton-activated G protein-coupled receptor (GPCR) 4 (GPR4) is constitutively active at physiological pH, and GPR4 knockout protected dopaminergic neurons from caspase-dependent mitochondria-associated apoptosis. This study explored the role of GPR4 in a 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-treated mouse model of Parkinson's disease (PD). In mice, subchronic MPTP administration causes oxidative stress-induced apoptosis in the dopaminergic neurons of the substantia nigra pars compacta (SNpc), resulting in motor deficits. NE52-QQ57, a selective GPR4 antagonist, reduced dopaminergic neuronal loss in MPTP-treated mice, improving motor and memory functions. MPTP and NE52-QQ57 co-treatment in mice significantly decreased pro-apoptotic marker Bax protein levels and increased anti-apoptotic marker Bcl-2 protein levels in the SNpc and striatum. MPTP-induced caspase 3 activation and poly (ADP-ribose) polymerase (PARP) cleavage significantly decreased in the SNpc and striatum of mice co-treated with NE52-QQ57. MPTP and NE52-QQ57 co-treatment significantly increased tyrosine hydroxylase (TH)-positive cell numbers in the SNpc and striatum compared with MPTP alone. NE52-QQ57 and MPTP co-treatment improved rotarod and pole test-assessed motor performance and improved Y-maze test-assessed spatial memory. Our findings suggest GPR4 may represent a potential therapeutic target for PD, and GPR4 activation is involved in caspase-mediated neuronal apoptosis in the SNpc and striatum of MPTP-treated mice.
Keywords: GPR4 receptor; MPTP; PARP; Parkinson’s disease; apoptosis; caspase 3; neurodegeneration.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
GPR4 Knockout Improves the Neurotoxin-Induced, Caspase-Dependent Mitochondrial Apoptosis of the Dopaminergic Neuronal Cell.Int J Mol Sci. 2020 Oct 12;21(20):7517. doi: 10.3390/ijms21207517. Int J Mol Sci. 2020. PMID: 33053856 Free PMC article.
-
Neuroprotective Effects of the DPP4 Inhibitor Vildagliptin in In Vivo and In Vitro Models of Parkinson's Disease.Int J Mol Sci. 2022 Feb 21;23(4):2388. doi: 10.3390/ijms23042388. Int J Mol Sci. 2022. PMID: 35216503 Free PMC article.
-
Neuroprotective properties of icariin in MPTP-induced mouse model of Parkinson's disease: Involvement of PI3K/Akt and MEK/ERK signaling pathways.Phytomedicine. 2017 Feb 15;25:93-99. doi: 10.1016/j.phymed.2016.12.017. Epub 2016 Dec 29. Phytomedicine. 2017. PMID: 28190476
-
MPTP-induced mouse model of Parkinson's disease: A promising direction of therapeutic strategies.Bosn J Basic Med Sci. 2021 Aug 1;21(4):422-433. doi: 10.17305/bjbms.2020.5181. Bosn J Basic Med Sci. 2021. PMID: 33357211 Free PMC article. Review.
-
Neuropharmacological approach against MPTP (1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine)-induced mouse model of Parkinson's disease.Acta Neurobiol Exp (Wars). 2011;71(2):269-80. doi: 10.55782/ane-2011-1847. Acta Neurobiol Exp (Wars). 2011. PMID: 21731080 Review.
Cited by
-
A neurotherapeutic approach with Lacticaseibacillus rhamnosus E9 on gut microbiota and intestinal barrier in MPTP-induced mouse model of Parkinson's disease.Sci Rep. 2024 Jul 4;14(1):15460. doi: 10.1038/s41598-024-65061-w. Sci Rep. 2024. PMID: 38965287 Free PMC article.
-
The Roles of Proton-Sensing G-Protein-Coupled Receptors in Inflammation and Cancer.Genes (Basel). 2024 Sep 1;15(9):1151. doi: 10.3390/genes15091151. Genes (Basel). 2024. PMID: 39336742 Free PMC article. Review.
-
Infiltrating peripheral monocyte TREM-1 mediates dopaminergic neuron injury in substantia nigra of Parkinson's disease model mice.Cell Death Dis. 2025 Jan 14;16(1):18. doi: 10.1038/s41419-025-07333-5. Cell Death Dis. 2025. PMID: 39809747 Free PMC article.
-
Gut Microbial Alteration in MPTP Mouse Model of Parkinson Disease is Administration Regimen Dependent.Cell Mol Neurobiol. 2023 Aug;43(6):2815-2829. doi: 10.1007/s10571-023-01319-7. Epub 2023 Jan 28. Cell Mol Neurobiol. 2023. PMID: 36708421 Free PMC article.
-
Human microRNA-4433 (hsa-miR-4443) Targets 18 Genes to be a Risk Factor of Neurodegenerative Diseases.Curr Alzheimer Res. 2022;19(7):511-522. doi: 10.2174/1567205019666220805120303. Curr Alzheimer Res. 2022. PMID: 35929619 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

