PD-L1 Expression in Muscle Invasive Urothelial Carcinomas as Assessed via Immunohistochemistry: Correlations with Specific Clinical and Pathological Features, with Emphasis on Prognosis after Radical Cystectomy
- PMID: 33925149
- PMCID: PMC8146852
- DOI: 10.3390/life11050404
PD-L1 Expression in Muscle Invasive Urothelial Carcinomas as Assessed via Immunohistochemistry: Correlations with Specific Clinical and Pathological Features, with Emphasis on Prognosis after Radical Cystectomy
Abstract
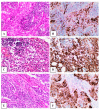
In the present study, we analyzed Programmed Death Ligand-1 (PD-L1) expression in radical cystectomy (RC) specimens from patients with muscle-invasive urothelial carcinoma (UC), in order to assess any correlations with specific clinicopathological features and its potential prognostic value. A multi-institutional study was performed within the departments of urology and pathology at the Mureș County Hospital, Romania, and Centre Hospitalier Lyon Sud, France. Sixty-nine patients with MIBC were included, for whom tumor histology (conventional versus histological variant/differentiation), tumor extension (T), lymph node involvement (N), and distant metastases (M) were recorded. PD-L1 immunostaining was performed using the 22C3 clone and was interpreted using the combined positive score (CPS) as recommended (Dako Agilent, Santa Clara, CA, USA). Positive PD-L1 immunostaining was more prevalent among UCs with squamous differentiation compared to conventional UCs and trended towards an improved OS (p = 0.366). We found the T stage to be a risk factor for poor survival in PD-L1-positive patients (HR 2.9, p = 0.021), along with the N stage in PD-L1-negative patients (HR 1.98, p = 0.007). No other clinicopathological factor was found to be significantly associated with PD-L1 positivity. Thus, we confirm the need for PD-L1 immunostaining prior to initiating immune checkpoint inhibitor therapy for a more accurate assessment of the patients' chances of responding to treatment.
Keywords: immune checkpoint inhibitors; lymphatic node status overall survival; muscle-invasive bladder cancer; tumor stage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Witjes J.A., Bruins M., Cathomas R., Compérat E., Cowan N.C., Gakis G., Hernández V., Lorch A., Ribal M.J., Thalmann G.N., et al. In: EAU Guidelines on Muscle-Invasive and Metastatic Bladder Cancer. Witjes J.A., Bruins M., Cathomas R., Compérat E., Cowan N.C., Gakis G., Hernández V., Lorch A., Ribal M.J., Thalmann G.N., et al., editors. EAU Guidelines Office; Arnhem, The Netherlands: 2020.
-
- Reis H., Serrette R., Posada J., Lu V., Chen Y.-B., Gopalan A., Fine S.W., Tickoo S.K., Sirintrapun S.J., Iyer G., et al. PD-L1 Expression in Urothelial Carcinoma with Predominant or Pure Variant Histology: Concordance Among 3 Commonly Used and Commercially Available Antibodies. Am. J. Surg. Pathol. 2019;43:920–927. doi: 10.1097/PAS.0000000000001264. - DOI - PMC - PubMed
-
- Bellmunt J., Mullane S.A., Werner L., Fay A.P., Callea M., Leow J.J., Taplin M.E., Choueiri T.K., Hodi F.S., Freeman G.J., et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann. Oncol. 2015;26:812–817. doi: 10.1093/annonc/mdv009. - DOI - PubMed
-
- Tretiakova M., Fulton R., Kocherginsky M., Long T., Ussakli C., Antic T., Gown A. Concordance study of PD-L1 expression in primary and metastatic bladder carcinomas: Comparison of four commonly used antibodies and RNA expression. Mod. Pathol. 2018;31:623–632. doi: 10.1038/modpathol.2017.188. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

