MED12-Related (Neuro)Developmental Disorders: A Question of Causality
- PMID: 33925166
- PMCID: PMC8146938
- DOI: 10.3390/genes12050663
MED12-Related (Neuro)Developmental Disorders: A Question of Causality
Abstract
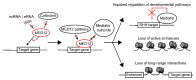
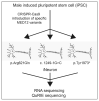
MED12 is a member of the Mediator complex that is involved in the regulation of transcription. Missense variants in MED12 cause FG syndrome, Lujan-Fryns syndrome, and Ohdo syndrome, as well as non-syndromic intellectual disability (ID) in hemizygous males. Recently, female patients with de novo missense variants and de novo protein truncating variants in MED12 were described, resulting in a clinical spectrum centered around ID and Hardikar syndrome without ID. The missense variants are found throughout MED12, whether they are inherited in hemizygous males or de novo in females. They can result in syndromic or nonsyndromic ID. The de novo nonsense variants resulting in Hardikar syndrome that is characterized by facial clefting, pigmentary retinopathy, biliary anomalies, and intestinal malrotation, are found more N-terminally, whereas the more C-terminally positioned variants are de novo protein truncating variants that cause a severe, syndromic phenotype consisting of ID, facial dysmorphism, short stature, skeletal abnormalities, feeding difficulties, and variable other abnormalities. This broad range of distinct phenotypes calls for a method to distinguish between pathogenic and non-pathogenic variants in MED12. We propose an isogenic iNeuron model to establish the unique gene expression patterns that are associated with the specific MED12 variants. The discovery of these patterns would help in future diagnostics and determine the causality of the MED12 variants.
Keywords: Hardikar syndome; MED12 variants; expression profile; iNeuronal model; intellectual disability; transcription regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Aranda-Orgilles B., Saldaña-Meyer R., Wang E., Trompouki E., Fassl A., Lau S., Mullenders J., Rocha P.P., Raviram R., Guillamot M., et al. MED12 Regulates HSC-Specific Enhancers Independently of Mediator Kinase Activity to Control Hematopoiesis. Cell Stem Cell. 2016;19:784–799. doi: 10.1016/j.stem.2016.08.004. - DOI - PMC - PubMed
-
- Chung H., Mao X., Wang H., Park Y.-J., Marcogliese P.C., Rosenfeld J.A., Burrage L.C., Liu P., Murdock D.R., Yamamoto S., et al. De Novo Variants in CDK19 Are Associated with a Syndrome Involving Intellectual Disability and Epileptic Encephalopathy. Am. J. Hum. Genet. 2020;106:717–725. doi: 10.1016/j.ajhg.2020.04.001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

