Skeletal Muscle Proteomic Profile Revealed Gender-Related Metabolic Responses in a Diet-Induced Obesity Animal Model
- PMID: 33925229
- PMCID: PMC8125379
- DOI: 10.3390/ijms22094680
Skeletal Muscle Proteomic Profile Revealed Gender-Related Metabolic Responses in a Diet-Induced Obesity Animal Model
Abstract
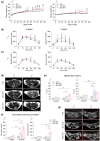
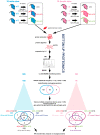
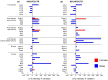
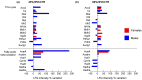
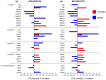
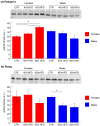
Obesity is a chronic, complex pathology associated with a risk of developing secondary pathologies, including cardiovascular diseases, cancer, type 2 diabetes (T2DM) and musculoskeletal disorders. Since skeletal muscle accounts for more than 70% of total glucose disposal, metabolic alterations are strictly associated with the onset of insulin resistance and T2DM. The present study relies on the proteomic analysis of gastrocnemius muscle from 15 male and 15 female C56BL/J mice fed for 14 weeks with standard, 45% or 60% high-fat diets (HFD) adopting a label-free LC-MS/MS approach followed by bioinformatic pathway analysis. Results indicate changes in males due to HFD, with increased muscular stiffness (Col1a1, Col1a2, Actb), fiber-type switch from slow/oxidative to fast/glycolytic (decreased Myh7, Myl2, Myl3 and increased Myh2, Mylpf, Mybpc2, Myl1), increased oxidative stress and mitochondrial dysfunction (decreased respiratory chain complex I and V and increased complex III subunits). At variance, females show few alterations and activation of compensatory mechanisms to counteract the increase of fatty acids. Bioinformatics analysis allows identifying upstream molecules involved in regulating pathways identified at variance in our analysis (Ppargc1a, Pparg, Cpt1b, Clpp, Tp53, Kdm5a, Hif1a). These findings underline the presence of a gender-specific response to be considered when approaching obesity and related comorbidities.
Keywords: insulin resistance; obesity; proteomics; sarcopenia; skeletal muscle.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- OECD Obesity Update 2017. [(accessed on 15 December 2020)]; Available online: http://www.oecd.org/health/obesity-update.htm.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

