Tissue Antioxidant Status and Lipid Peroxidation Are Related to Dietary Intake of n-3 Polyunsaturated Acids: A Rabbit Model
- PMID: 33925444
- PMCID: PMC8146135
- DOI: 10.3390/antiox10050681
Tissue Antioxidant Status and Lipid Peroxidation Are Related to Dietary Intake of n-3 Polyunsaturated Acids: A Rabbit Model
Abstract
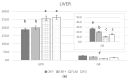
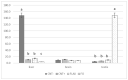
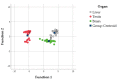
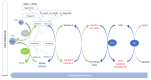
Polyunsaturated fatty acid (PUFA) metabolism and tissue distribution is modulated by the oxidation of these molecules. This research aimed to investigate the implication of dietary n-3 PUFA supplementation (precursor and long-chain PUFA) on the PUFA profile and oxidative status of the liver, testis, and brain of adult rabbit bucks. Twenty New Zealand White rabbit bucks were divided into four experimental groups (n = 5 per group) and were fed different diets for 110 days: control (CNT), standard diet containing 50 mg/kg alpha-tocopheryl acetate (vitamin E); CNT+, standard diet + 200 mg/kg vitamin E; FLAX, standard diet + 10% flaxseed + 200 mg/kg vitamin E; or FISH, standard diet + 3.5% fish oil + 200 mg/kg vitamin E. Antioxidants (enzymatic and non-enzymatic), oxidative status (malondialdehyde and isoprostanoids), and n-3 and n-6 PUFAs of tissues were analysed. A chain mechanism of oxidant/antioxidant molecules, which largely depended on the particular PUFA composition, was delineated in the different organs. The liver showed an oxidant/antioxidant profile and lipid pathways widely modulated by PUFA and vitamin E administration; on the other hand, the testis' oxidative profile rather than its lipid profile seemed to be particularly affected, an outcome opposite to that of the brain (modulation operated by dietary PUFA).
Keywords: brain; cellular antioxidants; isoprostanoids; liver; polyunsaturated fatty acids; rabbit; testis; vitamin E.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
LinkOut - more resources
Full Text Sources

