Combined Inhibition of TGF-β1-Induced EMT and PD-L1 Silencing Re-Sensitizes Hepatocellular Carcinoma to Sorafenib Treatment
- PMID: 33925488
- PMCID: PMC8123871
- DOI: 10.3390/jcm10091889
Combined Inhibition of TGF-β1-Induced EMT and PD-L1 Silencing Re-Sensitizes Hepatocellular Carcinoma to Sorafenib Treatment
Abstract
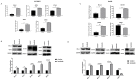
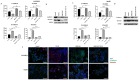
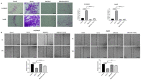
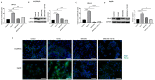
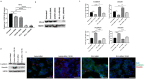
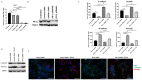
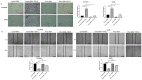
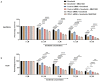
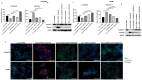
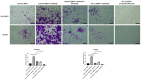
Hepatocellular carcinoma (HCC) is the most common type of primary hepatic malignancy. HCC is one of the leading causes of cancer deaths worldwide. The oral multi-tyrosine kinase inhibitor Sorafenib is the standard first-line therapy in patients with advanced unresectable HCC. Despite the significant survival benefit in HCC patients post treatment with Sorafenib, many patients had progressive disease as a result of acquiring drug resistance. Circumventing resistance to Sorafenib by exploring and targeting possible molecular mechanisms and pathways is an area of active investigation worldwide. Epithelial-to-mesenchymal transition (EMT) is a cellular process allowing epithelial cells to assume mesenchymal traits. HCC tumour cells undergo EMT to become immune evasive and develop resistance to Sorafenib treatment. Immune checkpoint molecules control immune escape in many tumours, including HCC. The aim of this study is to investigate whether combined inhibition of EMT and immune checkpoints can re-sensitise HCC to Sorafenib treatment. Post treatment with Sorafenib, HCC cells PLC/PRF/5 and Hep3B were monitored for induction of EMT and immune checkpoint molecules using quantitative reverse transcriptase (qRT)- PCR, western blot, immunofluorescence, and motility assays. The effect of combination treatment with SB431542, a specific inhibitor of the transforming growth factor (TGF)-β receptor kinase, and siRNA mediated knockdown of programmed cell death protein ligand-1 (PD-L1) on Sorafenib resistance was examined using a cell viability assay. We found that three days of Sorafenib treatment activated EMT with overexpression of TGF-β1 in both HCC cell lines. Following Sorafenib exposure, increase in the expression of PD-L1 and other immune checkpoints was observed. SB431542 blocked the TGF-β1-mediated EMT in HCC cells and also repressed PD-L1 expression. Likewise, knockdown of PD-L1 inhibited EMT. Moreover, the sensitivity of HCC cells to Sorafenib was enhanced by combining a blockade of EMT with SB431542 and knockdown of PD-L1 expression. Sorafenib-induced motility was attenuated with the combined treatment of SB431542 and PD-L1 knockdown. Our findings indicate that treatment with Sorafenib induces EMT and expression of immune checkpoint molecules, which contributes to Sorafenib resistance in HCC cells. Thus, the combination treatment strategy of inhibiting EMT and immune checkpoint molecules can re-sensitise HCC cells to Sorafenib.
Keywords: EMT; PD-L1; hepatocellular carcinoma; immune checkpoint; sorafenib.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Dual Targeting of Sorafenib-Resistant HCC-Derived Cancer Stem Cells.Curr Oncol. 2021 Jun 11;28(3):2150-2172. doi: 10.3390/curroncol28030200. Curr Oncol. 2021. PMID: 34208001 Free PMC article.
-
Immune checkpoint molecules are regulated by transforming growth factor (TGF)-β1-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma.Int J Med Sci. 2021 Apr 22;18(12):2466-2479. doi: 10.7150/ijms.54239. eCollection 2021. Int J Med Sci. 2021. PMID: 34104078 Free PMC article.
-
TNF‑α‑mediated epithelial‑to‑mesenchymal transition regulates expression of immune checkpoint molecules in hepatocellular carcinoma.Mol Med Rep. 2020 Apr;21(4):1849-1860. doi: 10.3892/mmr.2020.10991. Epub 2020 Feb 19. Mol Med Rep. 2020. PMID: 32319631 Free PMC article.
-
Treatment of advanced hepatocellular carcinoma: immunotherapy from checkpoint blockade to potential of cellular treatment.Transl Gastroenterol Hepatol. 2018 Nov 7;3:89. doi: 10.21037/tgh.2018.10.16. eCollection 2018. Transl Gastroenterol Hepatol. 2018. PMID: 30603725 Free PMC article. Review.
-
Hepatocellular Carcinoma: Etiology and Current and Future Drugs.J Clin Exp Hepatol. 2019 Mar-Apr;9(2):221-232. doi: 10.1016/j.jceh.2019.01.004. Epub 2019 Jan 25. J Clin Exp Hepatol. 2019. PMID: 31024205 Free PMC article. Review.
Cited by
-
Overcoming the therapeutic resistance of hepatomas by targeting the tumor microenvironment.Front Oncol. 2022 Nov 15;12:988956. doi: 10.3389/fonc.2022.988956. eCollection 2022. Front Oncol. 2022. PMID: 36457492 Free PMC article. Review.
-
The Roles of Epigenetic Regulation and the Tumor Microenvironment in the Mechanism of Resistance to Systemic Therapy in Hepatocellular Carcinoma.Int J Mol Sci. 2023 Feb 1;24(3):2805. doi: 10.3390/ijms24032805. Int J Mol Sci. 2023. PMID: 36769116 Free PMC article. Review.
-
Gadoxetic Acid-Enhanced Magnetic Resonance Imaging Features Can Predict Immune-Excluded Phenotype of Hepatocellular Carcinoma.Liver Cancer. 2024 Nov 13;14(3):271-285. doi: 10.1159/000542099. eCollection 2025 Jun. Liver Cancer. 2024. PMID: 40547958 Free PMC article.
-
Overexpression of PD-L1 is an Independent Predictor for Recurrence in HCC Patients Who Receive Sorafenib Treatment After Surgical Resection.Front Oncol. 2022 Jan 18;11:783335. doi: 10.3389/fonc.2021.783335. eCollection 2021. Front Oncol. 2022. PMID: 35117990 Free PMC article.
-
Dual Targeting of Sorafenib-Resistant HCC-Derived Cancer Stem Cells.Curr Oncol. 2021 Jun 11;28(3):2150-2172. doi: 10.3390/curroncol28030200. Curr Oncol. 2021. PMID: 34208001 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

