Nucleoside-Lipid-Based Nanoparticles for Phenazine Delivery: A New Therapeutic Strategy to Disrupt Hsp27-eIF4E Interaction in Castration Resistant Prostate Cancer
- PMID: 33925528
- PMCID: PMC8146835
- DOI: 10.3390/pharmaceutics13050623
Nucleoside-Lipid-Based Nanoparticles for Phenazine Delivery: A New Therapeutic Strategy to Disrupt Hsp27-eIF4E Interaction in Castration Resistant Prostate Cancer
Abstract
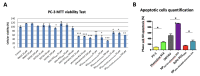
Heat shock protein 27 (Hsp27) has an established role in tumor progression and chemo-resistance of castration-resistant prostate cancer (CRPC). Hsp27 protects eukaryotic translation initiation factor 4E (eIF4E) from degradation, thereby maintaining survival during treatment. Phenazine derivative compound #14 was demonstrated to specifically disrupt Hsp27/eIF4E interaction and significantly delay castration-resistant tumor progression in prostate cancer xenografts. In the present work, various strategies of encapsulation of phenazine #14 with either DOTAU (N-[5'-(2',3'-dioleoyl)uridine]-N',N',N'-trimethylammonium tosylate) and DOU-PEG2000 (5'-PEG2000-2',3'-dioleoyluridine) nucleolipids (NLs) were developed in order to improve its solubilization, biological activity, and bioavailability. We observed that NLs-encapsulated phenazine #14-driven Hsp27-eIF4E interaction disruption increased cytotoxic effects on castration-resistant prostate cancer cell line and inhibited tumor growth in castration-resistant prostate cancer cell xenografted mice compared to phenazine #14 and NLs alone. Phenazine #14 NL encapsulation might represent an interesting nanostrategy for CRPC therapy.
Keywords: dialkoxyphenazine; nanoformulation; nucleolipid; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Petrylak D.P., Tangen C.M., Hussain M.H.A., Lara P.N., Jones J.A., Taplin M.E., Burch P.A., Berry D., Moinpour C., Kohli M., et al. Docetaxel and Estramustine Compared with Mitoxantrone and Prednisone for Advanced Refractory Prostate Cancer. N. Engl. J. Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. - DOI - PubMed
-
- James N.D., Sydes M.R., Clarke N.W., Mason M.D., Dearnaley D.P., Spears M.R., Ritchie A.W.S., Parker C.C., Russell J.M., Attard G., et al. Addition of Docetaxel, Zoledronic Acid, or Both to First-Line Long-Term Hormone Therapy in Prostate Cancer (STAMPEDE): Survival Results from an Adaptive, Multiarm, Multistage, Platform Randomised Controlled Trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

