MRD-Based Therapeutic Decisions in Genetically Defined Subsets of Adolescents and Young Adult Philadelphia-Negative ALL
- PMID: 33925541
- PMCID: PMC8123823
- DOI: 10.3390/cancers13092108
MRD-Based Therapeutic Decisions in Genetically Defined Subsets of Adolescents and Young Adult Philadelphia-Negative ALL
Abstract
In many clinical studies published over the past 20 years, adolescents and young adults (AYA) with Philadelphia chromosome negative acute lymphoblastic leukemia (Ph- ALL) were considered as a rather homogeneous clinico-prognostic group of patients suitable to receive intensive pediatric-like regimens with an improved outcome compared with the use of traditional adult ALL protocols. The AYA group was defined in most studies by an age range of 18-40 years, with some exceptions (up to 45 years). The experience collected in pediatric ALL with the study of post-induction minimal residual disease (MRD) was rapidly duplicated in AYA ALL, making MRD a widely accepted key factor for risk stratification and risk-oriented therapy with or without allogeneic stem cell transplantation and experimental new drugs for patients with MRD detectable after highly intensive chemotherapy. This combined strategy has resulted in long-term survival rates of AYA patients of 60-80%. The present review examines the evidence for MRD-guided therapies in AYA's Ph- ALL, provides a critical appraisal of current treatment pitfalls and illustrates the ways of achieving further therapeutic improvement according to the massive knowledge recently generated in the field of ALL biology and MRD/risk/subset-specific therapy.
Keywords: acute lymphoblastic leukemia; adolescents and young adults; minimal residual disease; risk stratification; risk-oriented therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
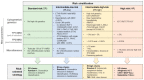
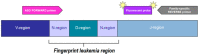
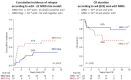
References
-
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER Cancer Statistic Review (1975–2017), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems. [(accessed on 17 December 2020)]; Available online: https://Seer.Cancer.Gov/Seerstat/
-
- Sant M., Allemani C., Tereanu C., De Angelis R., Capocaccia R., Visser O., Marcos-Gragera R., Maynadié M., Simonetti A., Lutz J.-M., et al. Incidence of Hematologic Malignancies in Europe by Morphologic Subtype: Results of the HAEMACARE Project. Blood. 2010;116:3724–3734. doi: 10.1182/blood-2010-05-282632. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

