Novel Organochlorinated Xerogels: From Microporous Materials to Ordered Domains
- PMID: 33925564
- PMCID: PMC8123792
- DOI: 10.3390/polym13091415
Novel Organochlorinated Xerogels: From Microporous Materials to Ordered Domains
Abstract
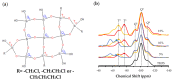
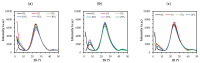
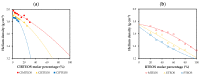
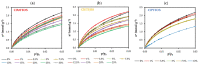
Hybrid silica xerogels combine the properties of organic and inorganic components in the same material, making them highly promising and versatile candidates for multiple applications. They can be tailored for specific purposes through chemical modifications, and the consequent changes in their structures warrant in-depth investigation. We describe the synthesis of three new series of organochlorinated xerogels prepared by co-condensation of tetraethyl orthosilicate (TEOS) and chloroalkyltriethoxysilane (ClRTEOS; R = methyl [M], ethyl [E], or propyl [P]) at different molar ratios. The influence of the precursors on the morphological and textural properties of the xerogels was studied using 29Si NMR (Nuclear Magnetic Resonance), FTIR (Fourier-Transform Infrared Spectroscopy), N2, and CO2 adsorption, XRD (X-ray Diffraction), and FE-SEM (Field-Emission Scanning Electron Microscopy). The structure and morphology of these materials are closely related to the nature and amount of the precursor, and their microporosity increases proportionally to the molar percentage of ClRTEOS. In addition, the influence of the chlorine atom was investigated through comparison with their non-chlorinated analogues (RTEOS, R = M, E, or P) prepared in previous studies. The results showed that a smaller amount of precursor was needed to detect ordered domains (ladders and T8 cages) in the local structure. The possibility of coupling self-organization with tailored porosity opens the way to novel applications for this type of organically modified silicates.
Keywords: ORMOSILs; TEOS; chloroalkyltriethoxysilane; hybrid materials; inductive effect; textural properties; xerogels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Judeinstein P., Sanchez C. Hybrid organic-inorganic materials: A land of multidisciplinarity. J. Mater. Chem. 1996;6:511–525. doi: 10.1039/JM9960600511. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

