On Iron Metabolism and Its Regulation
- PMID: 33925597
- PMCID: PMC8123811
- DOI: 10.3390/ijms22094591
On Iron Metabolism and Its Regulation
Abstract
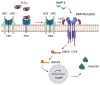
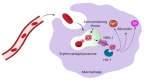
Iron is a critical metal for several vital biological processes. Most of the body's iron is bound to hemoglobin in erythrocytes. Iron from senescent red blood cells is recycled by macrophages in the spleen, liver and bone marrow. Dietary iron is taken up by the divalent metal transporter 1 (DMT1) in enterocytes and transported to portal blood via ferroportin (FPN), where it is bound to transferrin and taken up by hepatocytes, macrophages and bone marrow cells via transferrin receptor 1 (TfR1). While most of the physiologically active iron is bound hemoglobin, the major storage of most iron occurs in the liver in a ferritin-bound fashion. In response to an increased iron load, hepatocytes secrete the peptide hormone hepcidin, which binds to and induces internalization and degradation of the iron transporter FPN, thus controlling the amount of iron released from the cells into the blood. This review summarizes the key mechanisms and players involved in cellular and systemic iron regulation.
Keywords: hepcidin; iron; macrophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

