Hydrothermal Crystallization of Bismuth Oxychlorides (BiOCl) Using Different Shape Control Reagents
- PMID: 33925623
- PMCID: PMC8123882
- DOI: 10.3390/ma14092261
Hydrothermal Crystallization of Bismuth Oxychlorides (BiOCl) Using Different Shape Control Reagents
Abstract
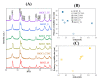
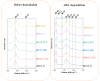
Bismuth oxychloride photocatalysts were obtained using solvothermal synthesis and different additives (CTAB-cetyltrimethylammonium bromide, CTAC-cetyltrimethylammonium chloride, PVP-polyvinylpyrrolidone, SDS-sodium dodecylsulphate, U-urea and TU-thiourea). The effect of the previously mentioned compounds was analyzed applying structural (primary crystallite size, crystal phase composition, etc.), morphological (particle geometry), optical (band gap energy) parameters, surface related properties (surface atoms' oxidation states), and the resulted photocatalytic activity. A strong dependency was found between the surface tension of the synthesis solutions and the overall morpho-structural parameters. The main finding was that the characteristics of the semiconductors can be tuned by modifying the surface tension of the synthesis mixture. It was observed after the photocatalytic degradation, that the white semiconductor turned to grey. Furthermore, we attempted to explain the gray color of BiOCl catalysts after the photocatalytic decompositions by Raman and XPS studies.
Keywords: bismuth oxychloride; photocatalytic activity; surface tension; surfactants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Sujuan W., Jiawei X., Jianguo S., Zachary D.H., Wen Z., Zhenzhong Y., Lin G., Xixiang Z., Yang S. Hydroxyl-dependent Evolution of Oxygen Vacancies Enables the Regeneration of BiOCl Photocatalyst. ACS Appl. Mater. Interfaces. 2017;9:16620–16626. - PubMed
-
- Zou Z., Xu H., Li D., Sun J., Xia D. Facile Preparation and Photocatalytic Activity of Oxygen Vacancy Rich BiOCl with {001} Exposed Reactive Facets. App. Sur. Sci. 2019;463:1011–1018. doi: 10.1016/j.apsusc.2018.09.025. - DOI
-
- Wang X., Liu X., Liu G., Zhang C., Liu G., Xu S., Cui P., Li D. Rapid Synthesis of BiOCl Graded Microspheres with Highly Exposed (110) Facets and Oxygen Vacancies at Room Temperature to Enhance Visible Light Photocatalytic Activity. Catal. Commun. 2019;130:105769. doi: 10.1016/j.catcom.2019.105769. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

