One-Time Foliar Application and Continuous Resupply via Roots Equally Improved the Growth and Physiological Response of B-Deficient Oilseed Rape
- PMID: 33925851
- PMCID: PMC8146809
- DOI: 10.3390/plants10050866
One-Time Foliar Application and Continuous Resupply via Roots Equally Improved the Growth and Physiological Response of B-Deficient Oilseed Rape
Abstract
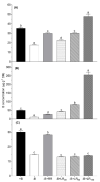
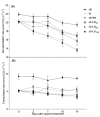
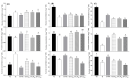
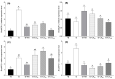
Oilseed rape (Brassica napus L.) is a high-boron (B)-demanding crop, and initially, normal growing plants might show B deficiency at advanced growth stages on soils with marginal B availability. Hence, we compared the effects of B resupply via roots and leaves on growth and physiological response, and relative expression of B transporters in B-deficient oilseed rape plants. Four-week-old plants initially grown with inadequate B (1 µM B for the first two weeks and 0.25 µM B for the next two weeks) were later grown either as such with 0.25 µM B, with 25 µM B in nutrient solution or foliar sprayed with 7 mL of 30, 60 and 150 mM B solution plant-1 as boric acid. Plants grown with 25 µM B in the nutrient solution from the beginning were included as adequate B treatment. Results showed that B resupply to B-deficient plants via roots and leaves (60 mM B) equally improved root and shoot dry matter, but not to the level of plants grown with adequate B supply. Foliar-applied 150 mM B proved toxic, causing leaf burn but not affecting dry matter. Resupply of B via roots increased B concentration in roots and leaves, while leaf-applied B did so only in leaves. Net carbon assimilation had a positive relationship with dry matter accumulation. Except for the highest foliar B level, B resupply via roots and leaves increased the accumulation of glucose, fructose and sucrose in leaves. Boron-deficient plants showed significant upregulation of BnaNIP5;1 in leaves and roots and of BnaBOR1;2 in roots. Boron resupply via roots reversed the B-deficiency-induced upregulation of BnaNIP5;1 in roots, whereas the expression of BnaBOR1;2 was reversed by both root and foliar B resupply. In leaves, B resupply by both methods reversed the expression of BnaNIP5;1 to the level of B-adequate plants. It is concluded that B resupply to B-deficient plants via roots and leaves equally but partially corrected B deficiency in B. napus grown in hydroponics.
Keywords: B resupply; B transporters; Brassica napus; boron; foliar application.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Boron uptake and distribution by oilseed rape (Brassica napus L.) as affected by different nitrogen forms under low and high boron supply.Plant Physiol Biochem. 2021 Apr;161:156-165. doi: 10.1016/j.plaphy.2021.02.009. Epub 2021 Feb 13. Plant Physiol Biochem. 2021. PMID: 33609922
-
Growth and Distribution of Boron in Oilseed Rape (Brassica napus L.) as Affected by Boron Supply.Plants (Basel). 2022 Oct 17;11(20):2746. doi: 10.3390/plants11202746. Plants (Basel). 2022. PMID: 36297770 Free PMC article.
-
Effects of boron foliar applications on vegetative and reproductive growth of sunflower.Ann Bot. 2003 Oct;92(4):565-70. doi: 10.1093/aob/mcg179. Epub 2003 Aug 21. Ann Bot. 2003. PMID: 12933368 Free PMC article.
-
Review: mechanisms for boron deficiency-mediated changes in plant water relations.Plant Sci. 2013 Apr;203-204:25-32. doi: 10.1016/j.plantsci.2012.12.012. Epub 2012 Dec 29. Plant Sci. 2013. PMID: 23415325 Review.
-
Role of magnesium in carbon partitioning and alleviating photooxidative damage.Physiol Plant. 2008 Aug;133(4):692-704. doi: 10.1111/j.1399-3054.2007.01042.x. Physiol Plant. 2008. PMID: 18724409 Review.
Cited by
-
Control of Early Blight Fungus (Alternaria alternata) in Tomato by Boric and Phenylboronic Acid.Antibiotics (Basel). 2022 Feb 28;11(3):320. doi: 10.3390/antibiotics11030320. Antibiotics (Basel). 2022. PMID: 35326783 Free PMC article.
-
Molecular, Metabolic and Physiological Responses to Boron Stress in Higher Plants.Plants (Basel). 2023 May 28;12(11):2136. doi: 10.3390/plants12112136. Plants (Basel). 2023. PMID: 37299115 Free PMC article.
References
-
- Shorrocks V.M. The occurrence and correction of boron deficiency. Plant Soil. 1997;193:121–148. doi: 10.1023/A:1004216126069. - DOI
-
- Marschner P. Marschner’s Mineral. Nutrition of Higher Plants. Elsevier Academic Press Inc.; San Diego, CA, USA: 2012.
-
- Dell B., Huang L.B. Physiological response of plants to low boron. Plant Soil. 1997;193:103–120. doi: 10.1023/A:1004264009230. - DOI
-
- Wimmer M.A., Goldberg S., Gupta U.C. Boron. In: Barker A., Pilbeam D., editors. Handbook of Plant Nutrition. 2nd ed. CRC Press; Boca Raton, FL, USA: 2015. pp. 305–346.
-
- Brown P.H., Shelp B.J. Boron mobility in plants. Plant Soil. 1997;193:85–101. doi: 10.1023/A:1004211925160. - DOI
LinkOut - more resources
Full Text Sources

