Molecular Characterization and Functional Analysis of Two Steroidogenic Genes TSPO and SMAD4 in Yellow Catfish
- PMID: 33925909
- PMCID: PMC8123483
- DOI: 10.3390/ijms22094505
Molecular Characterization and Functional Analysis of Two Steroidogenic Genes TSPO and SMAD4 in Yellow Catfish
Abstract
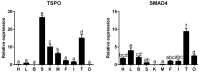
The steroid hormones are required for gonadal development in fish. The present study was undertaken to characterize the cDNA and promoter sequences of TSPO and SMAD4 genes in yellow catfish Pelteobagrus fulvidraco, explored the mRNA tissue expression and deciphered their promoter regions. Yellow catfish TSPO and SMAD4 shared the similar domains to the corresponding genes from other vertebrates. The TSPO and SMAD4 mRNAs were widely expressed in the detected tissues, but at different levels. Several transcription factors were predicted, such as Sp, GATA, AP1, SOX1, SRY, STAT, HNF4α, PPARγ, Pu.1 and FOXL2. PPARγ overexpression increased but STAT3 overexpression reduced TSPO promoter activity, and FOXL2 overexpression inhibited the promoter activity of TSPO and SMAD4. The site mutation and EMSA analysis indicated that TSPO promoter possessed STAT3 and FOXL2 sites. Overall, our provided the novel understanding into the transcriptionally regulatory mechanisms of TSPO and SMAD4 in fish.
Keywords: Pelteobagrus fulvidraco; molecular characterization; promoter; steroidogenesis-related genes; transcriptional regulation.
Conflict of interest statement
The authors disclosed no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

