Microbial and Genetic Resources for Cobalamin (Vitamin B12) Biosynthesis: From Ecosystems to Industrial Biotechnology
- PMID: 33926061
- PMCID: PMC8123684
- DOI: 10.3390/ijms22094522
Microbial and Genetic Resources for Cobalamin (Vitamin B12) Biosynthesis: From Ecosystems to Industrial Biotechnology
Abstract
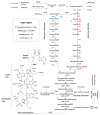
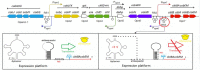
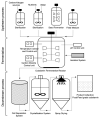
Many microbial producers of coenzyme B12 family cofactors together with their metabolically interdependent pathways are comprehensively studied and successfully used both in natural ecosystems dominated by auxotrophs, including bacteria and mammals, and in the safe industrial production of vitamin B12. Metabolic reconstruction for genomic and metagenomic data and functional genomics continue to mine the microbial and genetic resources for biosynthesis of the vital vitamin B12. Availability of metabolic engineering techniques and usage of affordable and renewable sources allowed improving bioprocess of vitamins, providing a positive impact on both economics and environment. The commercial production of vitamin B12 is mainly achieved through the use of the two major industrial strains, Propionobacterium shermanii and Pseudomonas denitrificans, that involves about 30 enzymatic steps in the biosynthesis of cobalamin and completely replaces chemical synthesis. However, there are still unresolved issues in cobalamin biosynthesis that need to be elucidated for future bioprocess improvements. In the present work, we review the current state of development and challenges for cobalamin (vitamin B12) biosynthesis, describing the major and novel prospective strains, and the studies of environmental factors and genetic tools effecting on the fermentation process are reported.
Keywords: auxotrophy; biosynthesis pathways and regulation; cobalamin biotechnology; cobamide-producing strains; coenzyme B12 family cofactors; genetic diversity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Guo M., Chen Y. Coenzyme cobalamin: Biosynthesis, overproduction and its application in dehalogenation-a review. Rev. Environ. Sci. Biotechnol. 2018;17:259–284. doi: 10.1007/s11157-018-9461-6. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

