Age-based targeting of biannual azithromycin distribution for child survival in Niger: an adaptive cluster-randomized trial protocol (AVENIR)
- PMID: 33926403
- PMCID: PMC8082631
- DOI: 10.1186/s12889-021-10824-7
Age-based targeting of biannual azithromycin distribution for child survival in Niger: an adaptive cluster-randomized trial protocol (AVENIR)
Abstract
Background: Biannual distribution of azithromycin to children 1-59 months old reduced mortality by 14% in a cluster-randomized trial. The World Health Organization has proposed targeting this intervention to the subgroup of children 1-11 months old to reduce selection for antimicrobial resistance. Here, we describe a trial designed to determine the impact of age-based targeting of biannual azithromycin on mortality and antimicrobial resistance.
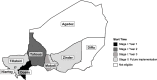
Methods: AVENIR is a cluster-randomized, placebo-controlled, double-masked, response-adaptive large simple trial in Niger. During the 2.5-year study period, 3350 communities are targeted for enrollment. In the first year, communities in the Dosso region will be randomized 1:1:1 to 1) azithromycin 1-11: biannual azithromycin to children 1-11 months old with placebo to children 12-59 months old, 2) azithromycin 1-59: biannual azithromycin to children 1-59 months old, or 3) placebo: biannual placebo to children 1-59 months old. Regions enrolled after the first year will be randomized with an updated allocation based on the probability of mortality in children 1-59 months in each arm during the preceding study period. A biannual door-to-door census will be conducted to enumerate the population, distribute azithromycin and placebo, and monitor vital status. Primary mortality outcomes are defined as all-cause mortality rate (deaths per 1000 person-years) after 2.5 years from the first enrollment in 1) children 1-59 months old comparing the azithromycin 1-59 and placebo arms, 2) children 1-11 months old comparing the azithromycin 1-11 and placebo arm, and 3) children 12-59 months in the azithromycin 1-11 and azithromycin 1-59 arms. In the Dosso region, 50 communities from each arm will be followed to monitor antimicrobial resistance. Primary resistance outcomes will be assessed after 2 years of distributions and include 1) prevalence of genetic determinants of macrolide resistance in nasopharyngeal samples from children 1-59 months old, and 2) load of genetic determinants of macrolide resistance in rectal samples from children 1-59 months old.
Discussion: As high-mortality settings consider this intervention, the results of this trial will provide evidence to support programmatic and policy decision-making on age-based strategies for azithromycin distribution to promote child survival.
Trial registration: This trial was registered on January 13, 2020 (clinicaltrials.gov: NCT04224987 ).
Keywords: Adaptive trial; Azithromycin; Cluster-randomized trial; Mass drug administration; Mortality.
Conflict of interest statement
None.
Figures

References
-
- GBD 2016 Child Mortality Collaborators Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1084–1150. doi: 10.1016/S0140-6736(17)31833-0. - DOI - PMC - PubMed
-
- United Nations Transforming our world . The 2030 agenda for sustainable development. New York: United Nations; 2015.
-
- International Trachoma Initiative Zithromax Shipments: Cumulative Treatments Shipped 2020 [Available from: https://www.trachoma.org/.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

