A heparin-rosuvastatin-loaded P(LLA-CL) nanofiber-covered stent inhibits inflammatory smooth-muscle cell viability to reduce in-stent stenosis and thrombosis
- PMID: 33926468
- PMCID: PMC8086342
- DOI: 10.1186/s12951-021-00867-8
A heparin-rosuvastatin-loaded P(LLA-CL) nanofiber-covered stent inhibits inflammatory smooth-muscle cell viability to reduce in-stent stenosis and thrombosis
Abstract
Background: An endovascular covered-stent has unique advantages in treating complex intracranial aneurysms; however, in-stent stenosis and late thrombosis have become the main factors affecting the efficacy of covered-stent treatment. Smooth-muscle-cell phenotypic modulation plays an important role in late in-stent stenosis and thrombosis. Here, we determined the efficacy of using covered stents loaded with drugs to inhibit smooth-muscle-cell phenotypic modulation and potentially lower the incidence of long-term complications.
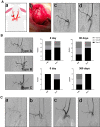
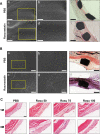
Methods: Nanofiber-covered stents were prepared using coaxial electrospinning, with the core solution prepared with 15% heparin and 20 µM rosuvastatin solution (400: 100 µL), and the shell solution prepared with 120 mg/mL hexafluoroisopropanol. We established a rabbit carotid-artery aneurysm model, which was treated with covered stents. Angiography and histology were performed to evaluate the therapeutic efficacy and incidence rate of in-stent stenosis and thrombosis. Phenotype, function, and inflammatory factors of smooth-muscle cells were studied to explore the mechanism of rosuvastatin action in smooth-muscle cells.
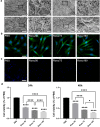
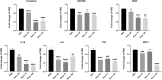
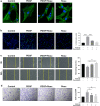
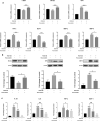
Result: Heparin-rosuvastatin-loaded nanofiber scaffold mats inhibited the proliferation of synthetic smooth-muscle cells, and the nanofiber-covered stent effectively treated aneurysms in the absence of notable in-stent stenosis. Additionally, in vitro experiments showed that rosuvastatin inhibited the smooth-muscle-cell phenotypic modulation of platelet-derived growth factor-BB induction and decreased synthetic smooth-muscle-cell viability, as well as secretion of inflammatory cytokines.
Conclusion: Rosuvastatin inhibited the abnormal proliferation of synthetic smooth-muscle cells, and heparin-rosuvastatin-loaded covered stents reduced the incidence of stenosis and late thrombosis, thereby improving the healing rates of stents used for aneurysm treatment.
Keywords: Intracranial aneurysm; Late thrombosis; Long-term arterial stenosis; Nanofiber-covered stent; Rosuvastatin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Greving JP, Wermer MJ, Brown RD, Jr, Morita A, Juvela S, Yonekura M, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13(1):59–66. doi: 10.1016/S1474-4422(13)70263-1. - DOI - PubMed
-
- Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267–1274. doi: 10.1016/S0140-6736(02)11314-6. - DOI - PubMed
-
- Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–817. doi: 10.1016/S0140-6736(05)67214-5. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 2017BR006/The Outstanding Academic Leaders Program of Shanghai Municipal Commission of Health and Family Planning
- 81571102/National Natural Science Foundation of China
- 81870911/National Natural Science Foundation of China
- 81801148/National Natural Science Foundation of China
- 2018SHZDZX01/Shanghai Municipal Science and Technology Major Project
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

