The diffuse-type tenosynovial giant cell tumor (dt-TGCT) patient journey: a prospective multicenter study
- PMID: 33926503
- PMCID: PMC8086070
- DOI: 10.1186/s13023-021-01820-6
The diffuse-type tenosynovial giant cell tumor (dt-TGCT) patient journey: a prospective multicenter study
Abstract
Background: Tenosynovial giant cell tumor (TGCT) is a rare, locally aggressive neoplasm arising from the synovium of joints, bursae, and tendon sheaths affecting small and large joints. It represents a wide spectrum ranging from minimally symptomatic to massively debilitating. Most findings to date are mainly from small, retrospective case series, and thus the morbidity and actual impact of this rare disease remain to be elucidated. This study prospectively explores the management of TGCT in tertiary sarcoma centers.
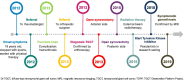
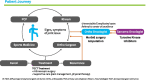
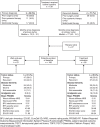
Methods: The TGCT Observational Platform Project registry was a multinational, multicenter, prospective observational study involving 12 tertiary sarcoma centers in 7 European countries, and 2 US sites. This study enrolled for 2 years all consecutive ≥ 18 years old patients, with histologically diagnosed primary or recurrent cases of diffuse-type TGCT. Patient demographic and clinical characteristics were collected at baseline and every 6 months for 24 months. Quality of life questionnaires (PROMIS-PF and EQ-5D) were also administered at the same time-points. Here we report baseline patient characteristics.
Results: 166 patients were enrolled between November 2016 and March 2019. Baseline characteristics were: mean age 44 years (mean age at disease onset: 39 years), 139/166 (83.7%) had prior treatment, 71/166 patients (42.8%) had ≥ 1 recurrence after treatment of their primary tumor, 76/136 (55.9%) visited a medical specialist ≥ 5 times, 66/116 (56.9%) missed work in the 24 months prior to baseline, and 17/166 (11.6%) changed employment status or retired prematurely due to disease burden. Prior treatment consisted of surgery (i.e., arthroscopic, open synovectomy) (128/166; 77.1%) and systemic treatments (52/166; 31.3%) with imatinib (19/52; 36.5%) or pexidartinib (27/52; 51.9%). Treatment strategies at baseline visits consisted mainly of watchful waiting (81/166; 48.8%), surgery (41/166; 24.7%), or targeted systemic therapy (37/166; 22.3%). Patients indicated for treatment reported more impairment compared to patients indicated for watchful waiting: worst stiffness NRS 5.16/3.44, worst pain NRS 6.13/5.03, PROMIS-PF 39.48/43.85, and EQ-5D VAS 66.54/71.85.
Conclusion: This study confirms that diffuse-type TGCT can highly impact quality of life. A prospective observational registry in rare disease is feasible and can be a tool to collect curated-population reflective data in orphan diseases. Name of registry: Tenosynovial Giant Cell Tumors (TGCT) Observational Platform Project (TOPP).
Trial registration number: NCT02948088. Date of registration: 10 October 2016. URL of Trial registry record: https://clinicaltrials.gov/ct2/show/NCT02948088?term=NCT02948088&draw=2 .
Keywords: Arthroscopy; Diagnosis; Diffuse TGCT; Patient journey; Surgery; Synovectomy; Systemic therapies; TOPP registry.
Conflict of interest statement
NMB reports consulting fees from Daiichi Sankyo, Zimmer Biomet, and Onkos Surgical. GS reports research funding to his institution (LUMC) from Daiichi Sankyo. JHH reports consulting fees from Daiichi Sankyo. EP served on an advisory board for Amgen, Daiichi Sankyo, Lilly, Eusa Pharma, Deciphera; research funding from Bristol-Myers Squibb, Pfizer, PharmaMar, and travel support from Lilly, PharmaMar, and Takeda. SB reports advisory board fees for Deciphera, Blueprint Medicines, ADC Therapeutics, Nanobiotix, Bayer, Lilly, Novartis, Exelixis, Daiichi Sankyo, and Roche; CME honoraria from PharmaMar, Lilly, and Novartis; research funding from Incyte, Blueprint Medicines, and Novartis; and travel support from PharmaMar. HG reports research funding to his institution (LUMC) from Daiichi Sankyo. ELS has served on a steering committee for Daiichi Sankyo Europe GmbH and an advisory board for Daiichi Sankyo Inc. JLB declares no competing interests. EMF, XY, and PL are employees for Daiichi Sankyo. MAJvdS reports research funding from Daiichi Sankyo. The TOPP Study Group reports the following conflicts of interests: BS reports limited research administrative compensation from Daiichi Sankyo; AL reports institution education grants from Johnson and Johnson, Alphamed, and Globus; JMB reports research funding from Lilly, PharmaMar, Eisai, Novartis, GSK, LIXTE, Karyopharm, Celgene, Pfizer, BMS, Blueprint, Deciphera, Nektar, Forma, Amgen, and Daiichi Sankyo; FG reports consulting fees from Amgen, stock ownership in Atlanthera, and honoraria from Deciphera; ALP and TC declare no competing interests.
Figures
Similar articles
-
Tenosynovial Giant Cell Tumor Observational Platform Project (TOPP) Registry: A 2-Year Analysis of Patient-Reported Outcomes and Treatment Strategies.Oncologist. 2023 Jun 2;28(6):e425-e435. doi: 10.1093/oncolo/oyad011. Oncologist. 2023. PMID: 36869793 Free PMC article.
-
A prospective real-world study of the diffuse-type tenosynovial giant cell tumor patient journey: A 2-year observational analysis.J Surg Oncol. 2022 Dec;126(8):1520-1532. doi: 10.1002/jso.27067. Epub 2022 Aug 25. J Surg Oncol. 2022. PMID: 36006054 Free PMC article.
-
The Patient Perspective on the Impact of Tenosynovial Giant Cell Tumors on Daily Living: Crowdsourcing Study on Physical Function and Quality of Life.Interact J Med Res. 2018 Feb 23;7(1):e4. doi: 10.2196/ijmr.9325. Interact J Med Res. 2018. PMID: 29475829 Free PMC article.
-
Pexidartinib for the treatment of adult patients with symptomatic tenosynovial giant cell tumor: safety and efficacy.Expert Rev Anticancer Ther. 2020 Jun;20(6):441-445. doi: 10.1080/14737140.2020.1757441. Epub 2020 Apr 22. Expert Rev Anticancer Ther. 2020. PMID: 32297819 Review.
-
Pexidartinib for the treatment of adult symptomatic patients with tenosynovial giant cell tumors.Expert Rev Clin Pharmacol. 2020 Jun;13(6):571-576. doi: 10.1080/17512433.2020.1771179. Epub 2020 Jun 1. Expert Rev Clin Pharmacol. 2020. PMID: 32478598 Review.
Cited by
-
Capturing Real-World Rare Disease Patient Journeys: Are Current Methodologies Sufficient for Informed Healthcare Decisions?J Eval Clin Pract. 2025 Feb;31(1):e70010. doi: 10.1111/jep.70010. J Eval Clin Pract. 2025. PMID: 39960234 Free PMC article.
-
Tenosynovial giant cell tumor and its differential diagnosis in children.Pediatr Radiol. 2025 Jul 19. doi: 10.1007/s00247-025-06338-8. Online ahead of print. Pediatr Radiol. 2025. PMID: 40681854 Review.
-
Localized and diffuse tenosynovial giant cell tumor: real-world results from a patient observational registry.Future Oncol. 2025 May;21(12):1501-1510. doi: 10.1080/14796694.2025.2488635. Epub 2025 Apr 8. Future Oncol. 2025. PMID: 40197108 Free PMC article.
-
Diffuse-type tenosynovial giant cell tumor between the suboccipital bone and posterior C1 arch: illustrative case.J Neurosurg Case Lessons. 2023 Aug 28;6(9):CASE23288. doi: 10.3171/CASE23288. Print 2023 Aug 28. J Neurosurg Case Lessons. 2023. PMID: 37728324 Free PMC article.
-
Tenosynovial giant cell tumours: experience at an Australian tertiary referral centre for musculoskeletal tumours with minimum two-year follow-up.Bone Jt Open. 2023 Nov 8;4(11):846-852. doi: 10.1302/2633-1462.411.BJO-2023-0116.R1. Bone Jt Open. 2023. PMID: 37935246 Free PMC article.
References
-
- de St. Aubain Somerhausen N, van de Rijn M. Tenosynovial giant cell tumour. In: Board WCoTE., editor. 5th World Health Organization classification of tumours of soft tissue and bone. 5. Lyon: IARC Press; 2020. pp. 133–136.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

