Radiofrequency ablation and chemotherapy versus chemotherapy alone for locally advanced pancreatic cancer (PELICAN): study protocol for a randomized controlled trial
- PMID: 33926539
- PMCID: PMC8082784
- DOI: 10.1186/s13063-021-05248-y
Radiofrequency ablation and chemotherapy versus chemotherapy alone for locally advanced pancreatic cancer (PELICAN): study protocol for a randomized controlled trial
Abstract
Background: Approximately 80% of patients with locally advanced pancreatic cancer (LAPC) are treated with chemotherapy, of whom approximately 10% undergo a resection. Cohort studies investigating local tumor ablation with radiofrequency ablation (RFA) have reported a promising overall survival of 26-34 months when given in a multimodal setting. However, randomized controlled trials (RCTs) investigating the effect of RFA in combination with chemotherapy in patients with LAPC are lacking.
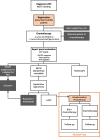
Methods: The "Pancreatic Locally Advanced Unresectable Cancer Ablation" (PELICAN) trial is an international multicenter superiority RCT, initiated by the Dutch Pancreatic Cancer Group (DPCG). All patients with LAPC according to DPCG criteria, who start with FOLFIRINOX or (nab-paclitaxel/)gemcitabine, are screened for eligibility. Restaging is performed after completion of four cycles of FOLFIRINOX or two cycles of (nab-paclitaxel/)gemcitabine (i.e., 2 months of treatment), and the results are assessed within a nationwide online expert panel. Eligible patients with RECIST stable disease or objective response, in whom resection is not feasible, are randomized to RFA followed by chemotherapy or chemotherapy alone. In total, 228 patients will be included in 16 centers in The Netherlands and four other European centers. The primary endpoint is overall survival. Secondary endpoints include progression-free survival, RECIST response, CA 19.9 and CEA response, toxicity, quality of life, pain, costs, and immunomodulatory effects of RFA.
Discussion: The PELICAN RCT aims to assess whether the combination of chemotherapy and RFA improves the overall survival when compared to chemotherapy alone, in patients with LAPC with no progression of disease following 2 months of systemic treatment.
Trial registration: Dutch Trial Registry NL4997 . Registered on December 29, 2015. ClinicalTrials.gov NCT03690323 . Retrospectively registered on October 1, 2018.
Keywords: Chemotherapy; Locally advanced pancreatic cancer; Overall survival; Radiofrequency ablation.
Conflict of interest statement
JWdG has received personal fees outside the submitted work from Bristol-Myers Squibb, Roche, Pierre-Fabre, Servier, MSD, and Novartis. RvH is a proctor for Intuitive, is part of the advisory board of Medtronics, and received an educational grant from Olympus outside the submitted work. IdH reports grants from Roche Pharmaceutical, QPS/RanD, and Medtronic, outside the submitted work. KvL reports personal fees and non-financial support from AngioDynamics, outside the submitted work. VEdM reports grants from Stichting Louise Vehmeijer and NWO and travel grants from Astellas and Neovii, outside the submitted work. MRM reports grants, personal fees, and non-financial support from Angiodynamics; grants and personal fees from Medtronic Covidien; and non-financial support from Cascination, outside the submitted work. NHM reports advisory board fees for her institution from BMS, Eli Lilly, Servier, and MSD. JdVG reports grants and non-financial support from Servier, outside the submitted work. JWW reports research grants from Servier, Halozyme, Novartis, Celgene, Astra Zeneca, Pfizer, Roche, Amgen, and Merck and a consulting/advisory role for Servier and Celgene. HCvS has received a research grant from the Dutch Cancer Society, during and outside the submitted work. MGB has received a research grant from the Dutch Cancer Society, during and outside the conduct of the study. IQM has received a research grant from the Dutch Cancer Society, during the conduct of the study. The other authors declare that they have no competing interests.
Figures
Similar articles
-
Nationwide implementation of the international multidisciplinary best-practice for locally advanced pancreatic cancer (PREOPANC-4): study protocol.BMC Cancer. 2025 Feb 19;25(1):299. doi: 10.1186/s12885-025-13554-w. BMC Cancer. 2025. PMID: 39972248 Free PMC article.
-
Triple modal treatment comprising with proton beam radiation, hyperthermia, and gemcitabine/nab-paclitaxel for locally advanced pancreatic cancer: a phase I/II study protocol (TT-LAP trial).BMC Cancer. 2023 Jul 4;23(1):624. doi: 10.1186/s12885-023-11110-y. BMC Cancer. 2023. PMID: 37403011 Free PMC article.
-
Comparison of combination therapies in the management of locally advanced pancreatic cancer: Induction chemotherapy followed by irreversible electroporation vs radiofrequency ablation.Cancer Med. 2020 Jul;9(13):4699-4710. doi: 10.1002/cam4.3119. Epub 2020 May 15. Cancer Med. 2020. PMID: 32410380 Free PMC article.
-
Multicenter randomized controlled trial and registry study to assess the safety and efficacy of the NanoKnife® system for the ablation of stage 3 pancreatic adenocarcinoma: overview of study protocols.BMC Cancer. 2021 Jul 7;21(1):785. doi: 10.1186/s12885-021-08474-4. BMC Cancer. 2021. PMID: 34233640 Free PMC article. Review.
-
Chemotherapy for pancreatic cancer.Presse Med. 2019 Mar;48(3 Pt 2):e159-e174. doi: 10.1016/j.lpm.2019.02.025. Epub 2019 Mar 15. Presse Med. 2019. PMID: 30879894 Review.
Cited by
-
Artificial intelligence for assessment of vascular involvement and tumor resectability on CT in patients with pancreatic cancer.Eur Radiol Exp. 2024 Feb 12;8(1):18. doi: 10.1186/s41747-023-00419-9. Eur Radiol Exp. 2024. PMID: 38342782 Free PMC article.
-
Image-Guided Percutaneous Ablation for Primary and Metastatic Tumors.Diagnostics (Basel). 2022 May 24;12(6):1300. doi: 10.3390/diagnostics12061300. Diagnostics (Basel). 2022. PMID: 35741109 Free PMC article. Review.
-
Pancreatic Cancer: Challenges and Opportunities in Locoregional Therapies.Cancers (Basel). 2022 Aug 31;14(17):4257. doi: 10.3390/cancers14174257. Cancers (Basel). 2022. PMID: 36077794 Free PMC article. Review.
-
Endoscopic Immuno-Oncology: A New Frontier in Treatment of Pancreatic Cancer.Cancers (Basel). 2025 Jun 23;17(13):2091. doi: 10.3390/cancers17132091. Cancers (Basel). 2025. PMID: 40647392 Free PMC article. Review.
-
State-of-the-Art and Upcoming Innovations in Pancreatic Cancer Care: A Step Forward to Precision Medicine.Cancers (Basel). 2023 Jun 30;15(13):3423. doi: 10.3390/cancers15133423. Cancers (Basel). 2023. PMID: 37444534 Free PMC article. Review.
References
-
- Latenstein AEJ, van der Geest LGM, Bonsing BA, Groot Koerkamp B, Haj Mohammad N, de Hingh IHJT, de Meijer VE, Molenaar IQ, van Santvoort H, van Tienhoven G, Verheij J, Vissers PAJ, de Vos-Geelen J, Busch OR, van Eijck C, van Laarhoven H, Besselink MG, Wilmink JW, Dutch Pancreatic Cancer Group Nationwide trends in incidence, treatment and survival of pancreatic ductal adenocarcinoma. Eur J Cancer. 2020;125:83–93. doi: 10.1016/j.ejca.2019.11.002. - DOI - PubMed
-
- Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. - DOI - PubMed
-
- Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, Zaniboni A, Ducreux M, Aitini E, Taïeb J, Faroux R, Lepere C, de Gramont A, GERCOR., GISCAD Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23(15):3509–3516. doi: 10.1200/JCO.2005.06.023. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical