Novel Botanical Therapeutic NB-02 Effectively Treats Alzheimer's Neuropathophysiology in an APP/PS1 Mouse Model
- PMID: 33926907
- PMCID: PMC8146489
- DOI: 10.1523/ENEURO.0389-20.2021
Novel Botanical Therapeutic NB-02 Effectively Treats Alzheimer's Neuropathophysiology in an APP/PS1 Mouse Model
Abstract
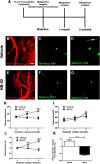
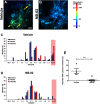
Alzheimer's disease (AD) is an incurable neurodegenerative disorder and a major cause of dementia. Some of the hallmarks of AD include presence of amyloid plaques in brain parenchyma, calcium dysregulation within individual neurons, and neuroinflammation. A promising therapeutic would reverse or stymie these pathophysiologies in an animal model of AD. We tested the effect of NB-02, previously known as DA-9803, a novel multimodal therapeutic, on amyloid deposition, neuronal calcium regulation and neuroinflammation in 8- to 10-month-old APP/PS1 mice, an animal model of AD. In vivo multiphoton microscopy revealed that two-month-long administration of NB-02 halted amyloid plaque deposition and cleared amyloid in the cortex. Postmortem analysis verified NB-02-dependent decrease in plaque deposition in the cortex as well as hippocampus. Furthermore, drug treatment reversed neuronal calcium elevations, thus restoring neuronal function. Finally, NB-02 restored spine density and transformed the morphology of astrocytes as well as microglia to a more phagocytic state, affecting neuroinflammation. NB-02 was effective at reversing AD neuropathophysiology in an animal model. Therefore, in addition to serving as a promising preventative agent, NB-02 holds potential as a treatment for AD in the clinic.
Keywords: Alzheimer’s disease; astrocyte; neuron; therapy.
Copyright © 2021 Lee et al.
Figures





Similar articles
-
Novel botanical drug DA-9803 prevents deficits in Alzheimer's mouse models.Alzheimers Res Ther. 2018 Jan 29;10(1):11. doi: 10.1186/s13195-018-0338-2. Alzheimers Res Ther. 2018. PMID: 29378621 Free PMC article.
-
Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer's disease.Neuropharmacology. 2014 Jan;76 Pt A:57-67. doi: 10.1016/j.neuropharm.2013.08.005. Epub 2013 Aug 21. Neuropharmacology. 2014. PMID: 23973293
-
The effect of focal brain injury on beta-amyloid plaque deposition, inflammation and synapses in the APP/PS1 mouse model of Alzheimer's disease.Exp Neurol. 2015 May;267:219-29. doi: 10.1016/j.expneurol.2015.02.034. Epub 2015 Mar 4. Exp Neurol. 2015. PMID: 25747037
-
ApoA-I deficiency increases cortical amyloid deposition, cerebral amyloid angiopathy, cortical and hippocampal astrogliosis, and amyloid-associated astrocyte reactivity in APP/PS1 mice.Alzheimers Res Ther. 2019 May 13;11(1):44. doi: 10.1186/s13195-019-0497-9. Alzheimers Res Ther. 2019. PMID: 31084613 Free PMC article.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
Cited by
-
Compensatory enhancement of orexinergic system functionality induced by amyloid-β protein: a neuroprotective response in Alzheimer's disease.Front Physiol. 2025 Mar 24;16:1529981. doi: 10.3389/fphys.2025.1529981. eCollection 2025. Front Physiol. 2025. PMID: 40196718 Free PMC article.
-
Optogenetic Targeting of Astrocytes Restores Slow Brain Rhythm Function and Slows Alzheimer's Disease Pathology.Res Sq [Preprint]. 2023 Apr 25:rs.3.rs-2813056. doi: 10.21203/rs.3.rs-2813056/v1. Res Sq. 2023. Update in: Sci Rep. 2023 Aug 11;13(1):13075. doi: 10.1038/s41598-023-40402-3. PMID: 37163040 Free PMC article. Updated. Preprint.
-
Optogenetic targeting of astrocytes restores slow brain rhythm function and slows Alzheimer's disease pathology.Sci Rep. 2023 Aug 11;13(1):13075. doi: 10.1038/s41598-023-40402-3. Sci Rep. 2023. PMID: 37567942 Free PMC article.
-
Astrocytes as a Therapeutic Target in Alzheimer's Disease-Comprehensive Review and Recent Developments.Int J Mol Sci. 2022 Nov 7;23(21):13630. doi: 10.3390/ijms232113630. Int J Mol Sci. 2022. PMID: 36362415 Free PMC article. Review.
-
Calcium sensor Yellow Cameleon 3.6 as a tool to support the calcium hypothesis of Alzheimer's disease.Alzheimers Dement. 2023 Sep;19(9):4196-4203. doi: 10.1002/alz.13111. Epub 2023 May 8. Alzheimers Dement. 2023. PMID: 37154246 Free PMC article. Review.
References
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, et al. (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21:383–421. 10.1016/S0197-4580(00)00124-X - DOI - PMC - PubMed
-
- Araque A (2006) Astrocyte-neuron signaling in the brain–implications for disease. Curr Opin Investig Drugs 7:619–624. - PubMed
-
- Bachstetter AD, Norris CM, Sompol P, Wilcock DM, Goulding D, Neltner JH, St.Clair D, Watterson DM, Van Eldik LJ (2012) Early stage drug treatment that normalizes proinflammatory cytokine production attenuates synaptic dysfunction in a mouse model that exhibits age-dependent progression of Alzheimer’s disease-related pathology. J Neurosci 32:10201–10210. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
