Stepwise pathogenic evolution of Mycobacterium abscessus
- PMID: 33926925
- PMCID: PMC7611193
- DOI: 10.1126/science.abb8699
Stepwise pathogenic evolution of Mycobacterium abscessus
Abstract
Although almost all mycobacterial species are saprophytic environmental organisms, a few, such as Mycobacterium tuberculosis, have evolved to cause transmissible human infection. By analyzing the recent emergence and spread of the environmental organism M. abscessus through the global cystic fibrosis population, we have defined key, generalizable steps involved in the pathogenic evolution of mycobacteria. We show that epigenetic modifiers, acquired through horizontal gene transfer, cause saltational increases in the pathogenic potential of specific environmental clones. Allopatric parallel evolution during chronic lung infection then promotes rapid increases in virulence through mutations in a discrete gene network; these mutations enhance growth within macrophages but impair fomite survival. As a consequence, we observe constrained pathogenic evolution while person-to-person transmission remains indirect, but postulate accelerated pathogenic adaptation once direct transmission is possible, as observed for M. tuberculosis Our findings indicate how key interventions, such as early treatment and cross-infection control, might restrict the spread of existing mycobacterial pathogens and prevent new, emergent ones.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
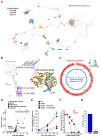

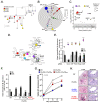

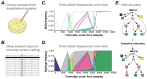
Comment in
-
Mycobacterium abscessus in cystic fibrosis.Science. 2021 Apr 30;372(6541):465-466. doi: 10.1126/science.abi5695. Science. 2021. PMID: 33926941 No abstract available.
References
-
- Griffith DE, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. - PubMed
-
- Richards CJ, Olivier KN. Nontuberculous Mycobacteria in Cystic Fibrosis. Semin Respir Crit Care Med. 2019;40:737–750. - PubMed
-
- Martiniano SL, Nick JA, Daley CL. Nontuberculous Mycobacterial Infections in Cystic Fibrosis. Thorac Surg Clin. 2019;29:95–108. - PubMed
-
- Haworth CS, et al. British Thoracic society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD) Thorax. 2017;72:ii1–ii64. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

