Diagnostic accuracy of a novel point-of-care urine lipoarabinomannan assay for the detection of tuberculosis among adult outpatients in Zambia: a prospective cross-sectional study
- PMID: 33926972
- PMCID: PMC8631000
- DOI: 10.1183/13993003.03999-2020
Diagnostic accuracy of a novel point-of-care urine lipoarabinomannan assay for the detection of tuberculosis among adult outpatients in Zambia: a prospective cross-sectional study
Abstract
Background: A novel, rapid, point-of-care urine-based lipoarabinomannan assay (Fujifilm SILVAMP TB LAM ("FujiLAM")) has previously demonstrated substantially higher sensitivity for tuberculosis (TB) compared with the commercially available Determine TB LAM assay using biobanked specimens. However, FujiLAM has not been prospectively evaluated using fresh urine specimens. Therefore, we determined the diagnostic accuracy of FujiLAM among HIV-positive and HIV-negative outpatients with presumptive TB in Zambia.
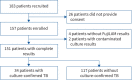
Methods: Adult (≥18 years old) presumptive TB patients presenting to two outpatient public health facilities in Lusaka were included. All patients submitted sputa samples for smear microscopy, Xpert MTB/RIF and mycobacterial culture, and urine samples for the FujiLAM assay. Microbiologically confirmed TB was defined by the detection of Mycobacterium tuberculosis in sputum using culture; this served as the reference standard to assess the diagnostic accuracy of FujiLAM.
Results: 151 adults with paired sputum microbiological tests and urine FujiLAM results were included; 45% were HIV-positive. Overall, 34 out of 151 (23%) patients had culture-confirmed pulmonary TB. The overall sensitivity and specificity of FujiLAM was 77% (95% CI 59-89%) and 92% (95% CI 86-96%), respectively. FujiLAM's sensitivity among HIV-positive patients was 75% (95% CI 43-95%) compared with 75% (95% CI 51-91%) among HIV-negative patients. The sensitivity of FujiLAM in patients with smear-positive, confirmed pulmonary TB was 87% (95% CI 60-98%) compared with 68% (95% CI 43-87%) among patients with smear-negative, confirmed pulmonary TB.
Conclusions: FujiLAM demonstrated high sensitivity for the detection of TB among both HIV-positive and HIV-negative adults, and also demonstrated good specificity despite the lack of systematic extrapulmonary sampling to inform a comprehensive microbiological reference standard.
Copyright ©The authors 2021.
Conflict of interest statement
Conflict of interest: M. Muyoyeta has nothing to disclose. Conflict of interest: A.D. Kerkhoff has nothing to disclose. Conflict of interest: L. Chilukutu has nothing to disclose. Conflict of interest: E. Moreau reports working for FIND. FIND is a non-for-profit foundation, whose mission is to find diagnostic solutions to overcome diseases of poverty in LMICs. It works closely with the private and public sectors and receives funding from some of its industry partners (no funding received from Cepheid for the development or the evaluation of Xpert). It has organisational firewalls to protect it against any undue influences in its work or the publication of its findings. All industry partnerships are subject to review by an independent scientific advisory committee or another independent review body, based on due diligence, TTPs and public sector requirements. FIND catalyses product development, leads evaluations, takes positions and accelerates access to tools identified as serving its mission. It provides indirect support to industry (e.g. access to open specimen banks, a clinical trial platform, technical support, expertise, laboratory capacity strengthening in LMICs) to facilitate the development and use of products in these areas. FIND also supports the evaluation of publicly prioritised TB assays and the implementation of WHO-approved (guidance and PQ) assays using donor grants. In order to carry out test evaluations, FIND has product evaluation agreements with several private sector companies for TB and other diseases, which strictly define its independence and neutrality vis-à-vis the companies whose products get evaluated and describes roles and responsibilities. Conflict of interest: S.G. Schumacher reports working for FIND. FIND is a non-for-profit foundation, whose mission is to find diagnostic solutions to overcome diseases of poverty in LMICs. It works closely with the private and public sectors and receives funding from some of its industry partners. It has organisational firewalls to protect it against any undue influences in its work or the publication of its findings. All industry partnerships are subject to review by an independent scientific advisory committee or another independent review body, based on due diligence, TPPs and public sector requirements. FIND catalyses product development, leads evaluations, takes positions and accelerates access to tools identified as serving its mission. It provides indirect support to industry (e.g. access to open specimen banks, a clinical trial platform, technical support, expertise, laboratory capacity strengthening in LMICs) to facilitate the development and use of products in these areas. FIND also supports the evaluation of prioritised assays and the early stages of implementation of WHO-approved (guidance and PQ) assays using donor grants. In order to carry out test validations and evaluations, FIND has product evaluation agreements with several private sector companies for the diseases FIND works in which strictly define its independence and neutrality vis-à-vis the companies whose products get evaluated, and describes roles and responsibilities. Conflict of interest: M. Ruhwald has nothing to disclose.
Figures



References
-
- World Health Organization . High priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting. 2014. www.who.int/tb/publications/tpp_report/en Date last accessed: 24 April 2021.
-
- World Health Organization . Lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis of active tuberculosis in people living with HIV: policy update 2019. 2019. www.who.int/tb/publications/2019/LAMPolicyUpdate2019/en Date last accessed: 24 April 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
