Ibrutinib combinations in CLL therapy: scientific rationale and clinical results
- PMID: 33927183
- PMCID: PMC8085243
- DOI: 10.1038/s41408-021-00467-7
Ibrutinib combinations in CLL therapy: scientific rationale and clinical results
Abstract
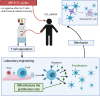
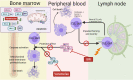
Ibrutinib has revolutionized the treatment of chronic lymphocytic leukemia (CLL). This drug irreversibly inhibits Bruton tyrosine kinase (BTK) by covalently binding to the C481 residue in the BTK kinase domain. BTK is a pivotal protein for B cell receptor signaling and tissue homing of CLL cells. Preclinical investigations have established the importance of the B cell receptor pathway in the maintenance and survival of normal and malignant B cells, underscoring the importance of targeting this axis for CLL. Clinical trials demonstrated overall and progression-free survival benefit with ibrutinib in multiple CLL subgroups, including patients with relapsed or refractory disease, patients with 17p deletion, elderly patients, and treatment-naïve patients. Consequently, ibrutinib was approved by the US Food and Drug Administration for newly diagnosed and relapsed disease. Ibrutinib has transformed the treatment of CLL; however, several limitations have been identified, including low complete remission rates, development of resistance, and uncommon substantial toxicities. Further, ibrutinib must be used until disease progression, which imposes a financial burden on patients and society. These limitations were the impetus for the development of ibrutinib combinations. Four strategies have been tested in recent years: combinations of ibrutinib with immunotherapy, chemoimmunotherapy, cell therapy, and other targeted therapy. Here, we review the scientific rationale for and clinical outcome of each strategy. Among these strategies, ibrutinib with targeted agent venetoclax results in high complete response rates and, importantly, high rates of undetectable minimal residual disease. Although we concentrate here on ibrutinib, similar combinations are expected or ongoing with acalabrutinib, tirabrutinib, and zanubrutinib, second-generation BTK inhibitors. Future investigations will focus on the feasibility of discontinuing ibrutinib combinations after a defined time; the therapeutic benefit of adding a third agent to ibrutinib-containing combinations; and profiling of resistant clones that develop after combination treatment. A new standard of care for CLL is expected to emerge from these investigations.
Conflict of interest statement
V.G. has received research grants from Pharmacyclics, Acerta, AbbVie, Gilead, Sunesis, Infinity, Loxo Oncology, and ClearCreek Bio. N.T. reports no competing financial interests.
Figures


References
-
- Tam C, et al. The BTK inhibitor, Bgb-3111, is safe, tolerable, and highly active in patients with relapsed/ refractory B-cell malignancies: initial report of a phase 1 first-in-human trial. Blood. 2015;126:832–832. doi: 10.1182/blood.V126.23.832.832. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

