CAR T-cell therapy in multiple myeloma: more room for improvement
- PMID: 33927192
- PMCID: PMC8085238
- DOI: 10.1038/s41408-021-00469-5
CAR T-cell therapy in multiple myeloma: more room for improvement
Abstract
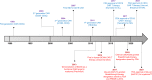
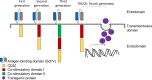
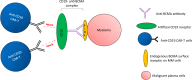
The emergence of various novel therapies over the last decade has changed the therapeutic landscape for multiple myeloma. While the clinical outcomes have improved significantly, the disease remains incurable, typically in patients with relapsed and refractory disease. Chimeric antigen receptor (CAR) T-cell therapies have achieved remarkable clinical success in B-cell malignancies. This scope of research has more recently been extended to the field of myeloma. While B-cell maturation antigen (BCMA) is currently the most well-studied CAR T antigen target in this disease, many other antigens are also undergoing intensive investigations. Some studies have shown encouraging results, whereas some others have demonstrated unfavorable results due to reasons such as toxicity and lack of clinical efficacy. Herein, we provide an overview of CAR T-cell therapies in myeloma, highlighted what has been achieved over the past decade, including the latest updates from ASH 2020 and discussed some of the challenges faced. Considering the current hits and misses of CAR T therapies, we provide a comprehensive analysis on the current manufacturing technologies, and deliberate on the future of CAR T-cell domain in MM.
Conflict of interest statement
The authors declare no competing interests.
Figures
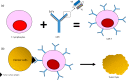

References
-
- Kumar SK, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15:1503–12. doi: 10.1016/S1470-2045(14)71125-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

