Calcium carbonate nanoparticles stimulate cancer cell reprogramming to suppress tumor growth and invasion in an organ-on-a-chip system
- PMID: 33927272
- PMCID: PMC8084943
- DOI: 10.1038/s41598-021-88687-6
Calcium carbonate nanoparticles stimulate cancer cell reprogramming to suppress tumor growth and invasion in an organ-on-a-chip system
Abstract
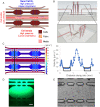
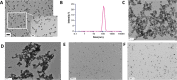
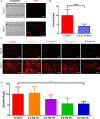
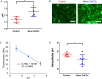
The acidic microenvironment of solid tumors induces the propagation of highly invasive and metastatic phenotypes. However, simulating these conditions in animal models present challenges that confound the effects of pH modulators on tumor progression. To recapitulate the tumor microenvironment and isolate the effect of pH on tumor viability, we developed a bifurcated microfluidic device that supports two different cell environments for direct comparison. RFP-expressing breast cancer cells (MDA-MB-231) were cultured in treatment and control chambers surrounded by fibrin, which received acid-neutralizing CaCO3 nanoparticles (nanoCaCO3) and cell culture media, respectively. Data analysis revealed that nanoCaCO3 buffered the pH within the normal physiological range and inhibited tumor cell proliferation compared to the untreated control (p < 0.05). Co-incubation of cancer cells and fibroblasts, followed by nanoCaCO3 treatment showed that the nanoparticles selectively inhibited the growth of the MDA-MB-231 cells and reduced cellular migration of these cells with no impact on the fibroblasts. Sustainable decrease in the intracellular pH of cancer cells treated with nanoCaCO3 indicates that the extracellular pH induced cellular metabolic reprogramming. These results suggest that the nanoCaCO3 can restrict the aggressiveness of tumor cells without affecting the growth and behavior of the surrounding stromal cells.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989;49:6449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 CA199092/CA/NCI NIH HHS/United States
- S10 OD027042/OD/NIH HHS/United States
- P50 CA094056/CA/NCI NIH HHS/United States
- R01 CA171651/CA/NCI NIH HHS/United States
- T32 GM007200/GM/NIGMS NIH HHS/United States
- S10 OD020129/OD/NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- R01 EB030987/EB/NIBIB NIH HHS/United States
- R01 EB021048/EB/NIBIB NIH HHS/United States
- F30 CA189435/CA/NCI NIH HHS/United States
- R01 CA260855/CA/NCI NIH HHS/United States
- S10 OD016237/OD/NIH HHS/United States
- R50 CA211481/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

