Inducible knockout of Clec16a in mice results in sensory neurodegeneration
- PMID: 33927318
- PMCID: PMC8084945
- DOI: 10.1038/s41598-021-88895-0
Inducible knockout of Clec16a in mice results in sensory neurodegeneration
Abstract
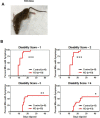
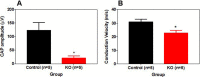
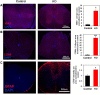
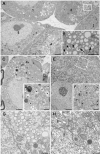
CLEC16A has been shown to play a role in autophagy/mitophagy processes. Additionally, genetic variants in CLEC16A have been implicated in multiple autoimmune diseases. We generated an inducible whole-body knockout, Clec16aΔUBC mice, to investigate the loss of function of CLEC16A. The mice exhibited a neuronal phenotype including tremors and impaired gait that rapidly progressed to dystonic postures. Nerve conduction studies and pathological analysis revealed loss of sensory axons that are associated with this phenotype. Activated microglia and astrocytes were found in regions of the CNS. Several mitochondrial-related proteins were up- or down-regulated. Upregulation of interferon stimulated gene 15 (IGS15) were observed in neuronal tissues. CLEC16A expression inversely related to IGS15 expression. ISG15 may be the link between CLEC16A and downstream autoimmune, inflammatory processes. Our results demonstrate that a whole-body, inducible knockout of Clec16a in mice results in an inflammatory neurodegenerative phenotype resembling spinocerebellar ataxia.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
CLEC16A-An Emerging Master Regulator of Autoimmunity and Neurodegeneration.Int J Mol Sci. 2023 May 4;24(9):8224. doi: 10.3390/ijms24098224. Int J Mol Sci. 2023. PMID: 37175930 Free PMC article. Review.
-
CLEC16A regulates splenocyte and NK cell function in part through MEK signaling.PLoS One. 2018 Sep 18;13(9):e0203952. doi: 10.1371/journal.pone.0203952. eCollection 2018. PLoS One. 2018. PMID: 30226884 Free PMC article.
-
JAK/STAT inhibitor therapy partially rescues the lipodystrophic autoimmune phenotype in Clec16a KO mice.Sci Rep. 2021 Apr 1;11(1):7372. doi: 10.1038/s41598-021-86493-8. Sci Rep. 2021. PMID: 33795715 Free PMC article.
-
Multiple sclerosis-associated CLEC16A controls HLA class II expression via late endosome biogenesis.Brain. 2015 Jun;138(Pt 6):1531-47. doi: 10.1093/brain/awv080. Epub 2015 Mar 29. Brain. 2015. PMID: 25823473 Free PMC article.
-
Neuronal injury in chronic CNS inflammation.Best Pract Res Clin Anaesthesiol. 2010 Dec;24(4):551-62. doi: 10.1016/j.bpa.2010.11.001. Epub 2010 Nov 29. Best Pract Res Clin Anaesthesiol. 2010. PMID: 21619866 Review.
Cited by
-
CLEC16A-An Emerging Master Regulator of Autoimmunity and Neurodegeneration.Int J Mol Sci. 2023 May 4;24(9):8224. doi: 10.3390/ijms24098224. Int J Mol Sci. 2023. PMID: 37175930 Free PMC article. Review.
-
CLEC16A interacts with retromer and TRIM27, and its loss impairs endosomal trafficking and neurodevelopment.Hum Genet. 2023 Mar;142(3):379-397. doi: 10.1007/s00439-022-02511-3. Epub 2022 Dec 20. Hum Genet. 2023. PMID: 36538041 Free PMC article.
-
SOCS-JAK-STAT inhibitors and SOCS mimetics as treatment options for autoimmune uveitis, psoriasis, lupus, and autoimmune encephalitis.Front Immunol. 2023 Oct 26;14:1271102. doi: 10.3389/fimmu.2023.1271102. eCollection 2023. Front Immunol. 2023. PMID: 38022642 Free PMC article. Review.
-
The genetics of autoimmune Addison disease: past, present and future.Nat Rev Endocrinol. 2022 Jul;18(7):399-412. doi: 10.1038/s41574-022-00653-y. Epub 2022 Apr 11. Nat Rev Endocrinol. 2022. PMID: 35411072 Review.
-
ISG15 and ISGylation in Human Diseases.Cells. 2022 Feb 4;11(3):538. doi: 10.3390/cells11030538. Cells. 2022. PMID: 35159348 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

