Theileria equi claudin like apicomplexan microneme protein contains neutralization-sensitive epitopes and interacts with components of the equine erythrocyte membrane skeleton
- PMID: 33927329
- PMCID: PMC8085155
- DOI: 10.1038/s41598-021-88902-4
Theileria equi claudin like apicomplexan microneme protein contains neutralization-sensitive epitopes and interacts with components of the equine erythrocyte membrane skeleton
Abstract
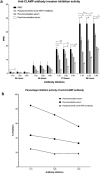
Theileria equi is a widely distributed apicomplexan parasite that causes severe hemolytic anemia in equid species. There is currently no effective vaccine for control of the parasite and understanding the mechanism that T. equi utilizes to invade host cells may be crucial for vaccine development. Unlike most apicomplexan species studied to date, the role of micronemes in T. equi invasion of host cells is unknown. We therefore assessed the role of the T. equi claudin-like apicomplexan microneme protein (CLAMP) in the invasion of equine erythrocytes as a first step towards understanding the role of this organelle in the parasite. Our findings show that CLAMP is expressed in the merozoite and intra-erythrocytic developmental stages of T. equi and in vitro neutralization experiments suggest that the protein is involved in erythrocyte invasion. Proteomic analyses indicate that CLAMP interacts with the equine erythrocyte α-and β- spectrin chains in the initial stages of T. equi invasion and maintains these interactions while also associating with the anion-exchange protein, tropomyosin 3, band 4.1 and cytoplasmic actin 1 after invasion. Additionally, serological analyses show that T. equi-infected horses mount robust antibody responses against CLAMP indicating that the protein is immunogenic and therefore represents a potential vaccine candidate.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Theileria equi RAP-1a and RAP-1b proteins contain immunoreactive epitopes and are suitable candidates for vaccine and diagnostics development.Int J Parasitol. 2022 May;52(6):385-397. doi: 10.1016/j.ijpara.2022.01.004. Epub 2022 Mar 19. Int J Parasitol. 2022. PMID: 35318949
-
Preparation of Monoclonal Antibody Against EMA-1 and Development of Rapid Serological Detection Method for Theileria equi Infection, Xinjiang, China.J Parasitol. 2020 Apr 1;106(2):283-290. doi: 10.1645/19-98. J Parasitol. 2020. PMID: 32296849
-
Evolution and diversity of the EMA families of the divergent equid parasites, Theileria equi and T. haneyi.Infect Genet Evol. 2019 Mar;68:153-160. doi: 10.1016/j.meegid.2018.12.020. Epub 2018 Dec 18. Infect Genet Evol. 2019. PMID: 30576837
-
Review of equine piroplasmosis.J Vet Intern Med. 2013 Nov-Dec;27(6):1334-46. doi: 10.1111/jvim.12168. Epub 2013 Aug 28. J Vet Intern Med. 2013. PMID: 24033559 Review.
-
Comparative Bioinformatics Analysis of Transcription Factor Genes Indicates Conservation of Key Regulatory Domains among Babesia bovis, Babesia microti, and Theileria equi.PLoS Negl Trop Dis. 2016 Nov 10;10(11):e0004983. doi: 10.1371/journal.pntd.0004983. eCollection 2016 Nov. PLoS Negl Trop Dis. 2016. PMID: 27832060 Free PMC article. Review.
Cited by
-
The claudin-like apicomplexan microneme protein is required for gliding motility and infectivity of Plasmodium sporozoites.PLoS Pathog. 2023 Mar 16;19(3):e1011261. doi: 10.1371/journal.ppat.1011261. eCollection 2023 Mar. PLoS Pathog. 2023. PMID: 36928686 Free PMC article.
-
Expression of IL-10 and TGF-β1 in horses experimentally infected with T. equi merozoites is associated with antibody production but not modulation of pro-inflammatory responses.Front Immunol. 2024 May 13;15:1370255. doi: 10.3389/fimmu.2024.1370255. eCollection 2024. Front Immunol. 2024. PMID: 38803499 Free PMC article.
References
-
- Carruthers V. B., S. L. D. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol.73, 114–123 (1997). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

