Metabolic pathways in obesity-related breast cancer
- PMID: 33927368
- PMCID: PMC10410950
- DOI: 10.1038/s41574-021-00487-0
Metabolic pathways in obesity-related breast cancer
Abstract
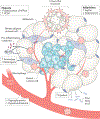
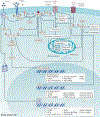
This Review focuses on the mechanistic evidence for a link between obesity, dysregulated cellular metabolism and breast cancer. Strong evidence now links obesity with the development of 13 different types of cancer, including oestrogen receptor-positive breast cancer in postmenopausal women. A number of local and systemic changes are hypothesized to support this relationship, including increased circulating levels of insulin and glucose as well as adipose tissue-derived oestrogens, adipokines and inflammatory mediators. Metabolic pathways of energy production and utilization are dysregulated in tumour cells and this dysregulation is a newly accepted hallmark of cancer. Dysregulated metabolism is also hypothesized to be a feature of non-neoplastic cells in the tumour microenvironment. Obesity-associated factors regulate metabolic pathways in both breast cancer cells and cells in the breast microenvironment, which provides a molecular link between obesity and breast cancer. Consequently, interventions that target these pathways might provide a benefit in postmenopausal women and individuals with obesity, a population at high risk of breast cancer.
Conflict of interest statement
Competing interests
The author declares no competing interests.
Figures

References
-
- Heer E. et al. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob. Health 8, e1027–e1037 (2020). - PubMed
-
- WHO. Obesity and Overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2020).
-
- Hales CM, Carroll MD, Fryar CD & Ogden CL Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief no. 360 (National Center for Health Statistics, 2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

