Free Chlorine and Peroxynitrite Alter the Capsid Structure of Human Norovirus GII.4 and Its Capacity to Bind Histo-Blood Group Antigens
- PMID: 33927710
- PMCID: PMC8076513
- DOI: 10.3389/fmicb.2021.662764
Free Chlorine and Peroxynitrite Alter the Capsid Structure of Human Norovirus GII.4 and Its Capacity to Bind Histo-Blood Group Antigens
Abstract
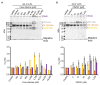
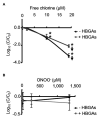
Human noroviruses (HuNoVs) are one of the leading causes of acute gastroenteritis worldwide. HuNoVs are frequently detected in water and foodstuffs. Free chlorine and peroxynitrite (ONOO-) are two oxidants commonly encountered by HuNoVs in humans or in the environment during their natural life cycle. In this study, we defined the effects of these two oxidants on GII.4 HuNoVs and GII.4 virus-like particles (VLPs). The impact on the capsid structure, the major capsid protein VP1 and the ability of the viral capsid to bind to histo-blood group antigens (HBGAs) following oxidative treatments were analyzed. HBGAs are attachment factors that promote HuNoV infection in human hosts. Overall, our results indicate that free chlorine acts on regions involved in the stabilization of VP1 dimers in VLPs and affects their ability to bind to HBGAs. These effects were confirmed in purified HuNoVs. Some VP1 cross-links also take place after free chlorine treatment, albeit to a lesser extent. Not only ONOO- mainly produced VP1 cross-links but can also dissociate VLPs depending on the concentration applied. Nevertheless, ONOO- has less effect on HuNoV particles.
Keywords: free chlorine; histo-blood group antigens; norovirus; peroxynitrite; viral protein; virus-like particles.
Copyright © 2021 Chassaing, Bastin, Robin, Majou, Belliot, de Rougemont, Boudaud and Gantzer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The effect of proteolytic enzymes and pH on GII.4 norovirus, during both interactions and non-interaction with Histo-Blood Group Antigens.Sci Rep. 2020 Oct 21;10(1):17926. doi: 10.1038/s41598-020-74728-z. Sci Rep. 2020. PMID: 33087754 Free PMC article.
-
Effect of natural ageing and heat treatments on GII.4 norovirus binding to Histo-Blood Group Antigens.Sci Rep. 2019 Oct 25;9(1):15312. doi: 10.1038/s41598-019-51750-4. Sci Rep. 2019. PMID: 31653918 Free PMC article.
-
Linear epitope binding antibodies against GII.3 Norovirus exhibit no histo-blood group antigens (HBGAs) blocking effects.Virus Genes. 2019 Jun;55(3):280-289. doi: 10.1007/s11262-019-01644-4. Epub 2019 Feb 6. Virus Genes. 2019. PMID: 30725444
-
Interaction between norovirus and Histo-Blood Group Antigens: A key to understanding virus transmission and inactivation through treatments?Food Microbiol. 2020 Dec;92:103594. doi: 10.1016/j.fm.2020.103594. Epub 2020 Jul 14. Food Microbiol. 2020. PMID: 32950136 Review.
-
The attachment factors and attachment receptors of human noroviruses.Food Microbiol. 2024 Oct;123:104591. doi: 10.1016/j.fm.2024.104591. Epub 2024 Jul 2. Food Microbiol. 2024. PMID: 39038896 Review.
Cited by
-
Chloride Enhances DNA Reactivity with Chlorine under Conditions Relevant to Water Treatment.Environ Sci Technol. 2022 Sep 20;56(18):13347-13356. doi: 10.1021/acs.est.2c03267. Epub 2022 Aug 26. Environ Sci Technol. 2022. PMID: 36027047 Free PMC article.
References
-
- Bastin G., Loison P., Vernex-Loset L., Dupire F., Challant J., Majou D., et al. . (2020). Structural organizations of Qβ and MS2 phages affect capsid protein modifications by oxidants hypochlorous acid and peroxynitrite. Front. Microbiol. 11:1157. 10.3389/fmicb.2020.01157, PMID: - DOI - PMC - PubMed
-
- Belliot G., Noel J. S., Li J. F., Seto Y., Humphrey C. D., Ando T., et al. . (2001). Characterization of capsid genes, expressed in the baculovirus system, of three new genetically distinct strains of “Norwalk-like viruses”. J. Clin. Microbiol. 39, 4288–4295. 10.1128/JCM.39.12.4288-4295.2001, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

