The Cancer Cell Dissemination Machinery as an Immunosuppressive Niche: A New Obstacle Towards the Era of Cancer Immunotherapy
- PMID: 33927723
- PMCID: PMC8076861
- DOI: 10.3389/fimmu.2021.654877
The Cancer Cell Dissemination Machinery as an Immunosuppressive Niche: A New Obstacle Towards the Era of Cancer Immunotherapy
Abstract
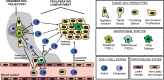
Although cancer immunotherapy has resulted in unpreceded survival benefits to subsets of oncology patients, accumulating evidence from preclinical animal models suggests that the immunosuppressive tumor microenvironment remains a detrimental factor limiting benefit for many patient subgroups. Recent efforts on lymphocyte-mediated immunotherapies are primarily focused on eliminating cancer foci at primary and metastatic sites, but few studies have investigated the impact of these therapies on the highly complex process of cancer cell dissemination. The metastatic cascade involves the directional streaming of invasive/migratory tumor cells toward specialized blood vessel intravasation gateways, called TMEM doorways, to the peripheral circulation. Importantly, this process occurs under the auspices of a specialized tumor microenvironment, herewith referred to as "Dissemination Trajectory", which is supported by an ample array of tumor-associated macrophages (TAMs), skewed towards an M2-like polarization spectrum, and which is also vital for providing microenvironmental cues for cancer cell invasion, migration and stemness. Based on pre-existing evidence from preclinical animal models, this article outlines the hypothesis that dissemination trajectories do not only support the metastatic cascade, but also embody immunosuppressive niches, capable of providing transient and localized immunosubversion cues to the migratory/invasive cancer cell subpopulation while in the act of departing from a primary tumor. So long as these dissemination trajectories function as "immune deserts", the migratory tumor cell subpopulation remains efficient in evading immunological destruction and seeding metastatic sites, despite administration of cancer immunotherapy and/or other cytotoxic treatments. A deeper understanding of the molecular and cellular composition, as well as the signaling circuitries governing the function of these dissemination trajectories will further our overall understanding on TAM-mediated immunosuppression and will be paramount for the development of new therapeutic strategies for the advancement of optimal cancer chemotherapies, immunotherapies, and targeted therapies.
Keywords: cancer immunotherapy; T cells; endothelial anergy; lymphocyte exclusion; lymphocyte exhaustion; macrophages; metastasis; tumor microenvironment.
Copyright © 2021 Asiry, Kim, Filippou, Sanchez, Entenberg, Marks, Oktay and Karagiannis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Hijacked Immune Cells in the Tumor Microenvironment: Molecular Mechanisms of Immunosuppression and Cues to Improve T Cell-Based Immunotherapy of Solid Tumors.Int J Mol Sci. 2021 May 27;22(11):5736. doi: 10.3390/ijms22115736. Int J Mol Sci. 2021. PMID: 34072260 Free PMC article. Review.
-
Immunosuppressive cells in tumor immune escape and metastasis.J Mol Med (Berl). 2016 May;94(5):509-22. doi: 10.1007/s00109-015-1376-x. Epub 2015 Dec 22. J Mol Med (Berl). 2016. PMID: 26689709 Review.
-
The Impact of the Tumor Microenvironment on Macrophage Polarization in Cancer Metastatic Progression.Int J Mol Sci. 2021 Jun 18;22(12):6560. doi: 10.3390/ijms22126560. Int J Mol Sci. 2021. PMID: 34207286 Free PMC article. Review.
-
Pushing Past the Blockade: Advancements in T Cell-Based Cancer Immunotherapies.Front Immunol. 2021 Nov 18;12:777073. doi: 10.3389/fimmu.2021.777073. eCollection 2021. Front Immunol. 2021. PMID: 34868044 Free PMC article. Review.
-
Immunological Consequences of Epithelial-Mesenchymal Transition in Tumor Progression.J Immunol. 2016 Aug 1;197(3):691-8. doi: 10.4049/jimmunol.1600458. J Immunol. 2016. PMID: 27431984 Free PMC article. Review.
Cited by
-
DeepST: identifying spatial domains in spatial transcriptomics by deep learning.Nucleic Acids Res. 2022 Dec 9;50(22):e131. doi: 10.1093/nar/gkac901. Nucleic Acids Res. 2022. PMID: 36250636 Free PMC article.
-
Mini-Review: Can the Metastatic Cascade Be Inhibited by Targeting CD147/EMMPRIN to Prevent Tumor Recurrence?Front Immunol. 2022 Mar 28;13:855978. doi: 10.3389/fimmu.2022.855978. eCollection 2022. Front Immunol. 2022. PMID: 35418981 Free PMC article. Review.
-
Assessment of MRI to estimate metastatic dissemination risk and prometastatic effects of chemotherapy.NPJ Breast Cancer. 2022 Sep 2;8(1):101. doi: 10.1038/s41523-022-00463-5. NPJ Breast Cancer. 2022. PMID: 36056005 Free PMC article.
-
Molecular understanding and clinical aspects of tumor-associated macrophages in the immunotherapy of renal cell carcinoma.J Exp Clin Cancer Res. 2024 Aug 22;43(1):242. doi: 10.1186/s13046-024-03164-y. J Exp Clin Cancer Res. 2024. PMID: 39169402 Free PMC article. Review.
-
Unsupervised spatially embedded deep representation of spatial transcriptomics.Genome Med. 2024 Jan 12;16(1):12. doi: 10.1186/s13073-024-01283-x. Genome Med. 2024. PMID: 38217035 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

