Metabolic Consequences of Efferocytosis and its Impact on Atherosclerosis
- PMID: 33927896
- PMCID: PMC8081385
- DOI: 10.20900/immunometab20210017
Metabolic Consequences of Efferocytosis and its Impact on Atherosclerosis
Abstract
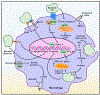
Billions of cells undergo apoptosis daily and are swiftly removed by macrophages through an evolutionarily conserved program termed "efferocytosis". Consequently, macromolecules within an apoptotic cell significantly burden a phagocyte with nutrients, such as lipids, oligonucleotides, and amino acids. In response to this nutrient overload, metabolic reprogramming must occur for the process of efferocytosis to remain non-phlogistic and to execute successive rounds of efferocytosis. The inability to undergo metabolic reprogramming after efferocytosis drives inflammation and impairs its resolution, often promoting many chronic inflammatory diseases. This is particularly evident for atherosclerosis, as metabolic reprogramming alters macrophage function in every stage of atherosclerosis, from the early formation of benign lesions to the progression of clinically relevant atheromas and during atherosclerosis regression upon aggressive lipid-lowering. This Review focuses on the metabolic pathways utilized upon apoptotic cell ingestion, the consequences of these metabolic pathways in macrophage function thereafter, and the role of metabolic reprogramming during atherosclerosis. Due to the growing interest in this new field, I introduce a new term, "efferotabolism", as a means to define the process by which macrophages break down, metabolize, and respond to AC-derived macromolecules. Understanding these aspects of efferotabolism will shed light on novel strategies to combat atherosclerosis and compromised inflammation resolution.
Keywords: atherosclerosis; efferocytosis; efferotabolism; macrophage; metabolism.
Conflict of interest statement
CONFLICTS OF INTEREST The author declares that there are no conflicts of interest.
Figures


References
-
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–31. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
