Systemic Administration of α7-Nicotinic Acetylcholine Receptor Ligands Does Not Improve Renal Injury or Behavior in Mice With Advanced Systemic Lupus Erythematosus
- PMID: 33928103
- PMCID: PMC8076522
- DOI: 10.3389/fmed.2021.642960
Systemic Administration of α7-Nicotinic Acetylcholine Receptor Ligands Does Not Improve Renal Injury or Behavior in Mice With Advanced Systemic Lupus Erythematosus
Abstract
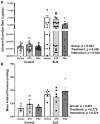
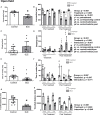
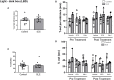
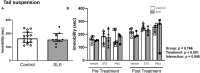
There is a critical need for safe treatment options to control inflammation in patients with systemic lupus erythematosus (SLE) since the inflammation contributes to morbidity and mortality in advanced disease. Endogenous neuroimmune mechanisms like the cholinergic anti-inflammatory pathway can be targeted to modulate inflammation, but the ability to manipulate such pathways and reduce inflammation and end organ damage has not been fully explored in SLE. Positive allosteric modulators (PAM) are pharmacological agents that inhibit desensitization of the nicotinic acetylcholine receptor (α7-nAChR), the main anti-inflammatory feature within the cholinergic anti-inflammatory pathway, and may augment α7-dependent cholinergic tone to generate therapeutic benefits in SLE. In the current study, we hypothesize that activating the cholinergic anti-inflammatory pathway at the level of the α7-nAChR with systemic administration of a partial agonist, GTS-21, and a PAM, PNU-120596, would reduce inflammation, eliminating the associated end organ damage in a mouse model of SLE with advanced disease. Further, we hypothesize that systemic α7 ligands will have central effects and improve behavioral deficits in SLE mice. Female control (NZW) and SLE mice (NZBWF1) were administered GTS-21 or PNU-120596 subcutaneously via minipumps for 2 weeks. We found that the increased plasma dsDNA autoantibodies, splenic and renal inflammation, renal injury and hypertension usually observed in SLE mice with advanced disease at 35 weeks of age were not altered by GTS-21 or PNU-120596. The anxiety-like behavior presented in SLE mice was also not improved by GTS-21 or PNU-120596. Although no significant beneficial effects of α7 ligands were observed in SLE mice at this advanced stage, we predict that targeting this receptor earlier in the pathogenesis of the disease may prove to be efficacious and should be addressed in future studies.
Keywords: SLE; behavior; cholinergic anti-inflammatory pathway; positive allosteric modulators; renal inflammation; renal injury.
Copyright © 2021 Morales, Young-Stubbs, Shimoura, Kem, Uteshev and Mathis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Cholinergic agonists reduce blood pressure in a mouse model of systemic lupus erythematosus.Physiol Rep. 2017 Apr;5(7):e13213. doi: 10.14814/phy2.13213. Physiol Rep. 2017. PMID: 28400502 Free PMC article.
-
Pharmacological potentiation of the efferent vagus nerve attenuates blood pressure and renal injury in a murine model of systemic lupus erythematosus.Am J Physiol Regul Integr Comp Physiol. 2018 Dec 1;315(6):R1261-R1271. doi: 10.1152/ajpregu.00362.2017. Epub 2018 Oct 17. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 30332305 Free PMC article.
-
α7 nicotinic acetylcholine receptor agonist GTS-21 attenuates ventilator-induced tumour necrosis factor-α production and lung injury.Br J Anaesth. 2011 Oct;107(4):559-66. doi: 10.1093/bja/aer202. Epub 2011 Jul 18. Br J Anaesth. 2011. PMID: 21771746
-
Role of the α7 Nicotinic Acetylcholine Receptor and RIC-3 in the Cholinergic Anti-inflammatory Pathway.Cent Nerv Syst Agents Med Chem. 2017;17(2):90-99. doi: 10.2174/1871524916666160829114533. Cent Nerv Syst Agents Med Chem. 2017. PMID: 27573666 Review.
-
Advances in the discovery of novel positive allosteric modulators of the alpha7 nicotinic acetylcholine receptor.Recent Pat CNS Drug Discov. 2007 Jun;2(2):99-106. doi: 10.2174/157488907780832751. Recent Pat CNS Drug Discov. 2007. PMID: 18221220 Review.
Cited by
-
The efficacy of an allosteric modulator of the alpha 7 nicotinic acetylcholine receptor in a murine model of stroke.Front Neurosci. 2025 Feb 12;19:1525975. doi: 10.3389/fnins.2025.1525975. eCollection 2025. Front Neurosci. 2025. PMID: 40012683 Free PMC article.
-
Targeted stimulation of the vagus nerve reduces renal injury in female mice with systemic lupus erythematosus.Auton Neurosci. 2023 Dec;250:103129. doi: 10.1016/j.autneu.2023.103129. Epub 2023 Nov 1. Auton Neurosci. 2023. PMID: 37950930 Free PMC article.
-
Optogenetic stimulation of basal forebrain cholinergic neurons prevents neuroinflammation and neuropsychiatric manifestations in pristane induced lupus mice.Behav Brain Funct. 2023 Jun 15;19(1):11. doi: 10.1186/s12993-023-00213-y. Behav Brain Funct. 2023. PMID: 37322485 Free PMC article.
-
Should Renal Inflammation Be Targeted While Treating Hypertension?Front Physiol. 2022 Jun 13;13:886779. doi: 10.3389/fphys.2022.886779. eCollection 2022. Front Physiol. 2022. PMID: 35770194 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

