Utility of Antigen-Based Rapid Diagnostic Test for Detection of SARS-CoV-2 Virus in Routine Hospital Settings
- PMID: 33928384
- PMCID: PMC8135470
- DOI: 10.1093/labmed/lmab033
Utility of Antigen-Based Rapid Diagnostic Test for Detection of SARS-CoV-2 Virus in Routine Hospital Settings
Abstract
Objective: This study aims to evaluate the performance of an antigen-based rapid diagnostic test (RDT) for the detection of the SARS-CoV-2 virus.
Methods: A cross-sectional study was conducted on 677 patients. Two nasopharyngeal swabs and 1 oropharyngeal swab were collected from patients. The RDT was performed onsite by a commercially available immune-chromatographic assay on the nasopharyngeal swab. The nasopharyngeal and oropharyngeal swabs were examined for SARS-CoV-2 RNA by real-time reverse-transcription quantitative polymerase chain reaction (RT-qPCR) assay.
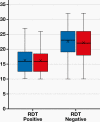
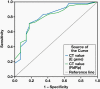
Results: The overall sensitivity of the SARS-CoV-2 RDT was 34.5% and the specificity was 99.8%. The positive predictive value and negative predictive value of the test were 96.6% and 91.5%, respectively. The detection rate of RDT in RT-qPCR positive results was high (45%) for cycle threshold values <25.
Conclusion: The utility of RDT is in diagnosing symptomatic patients and may not be particularly suited as a screening tool for patients with low viral load. The low sensitivity of RDT does not qualify its use as a single test in patients who test negative; RT-qPCR continues to be the gold standard test.
Keywords: COVID-19; RDT; RT-qPCR; rapid antigen test.
© American Society for Clinical Pathology, 2021. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

