Oligodendroglial GABAergic Signaling: More Than Inhibition!
- PMID: 33928492
- PMCID: PMC8275815
- DOI: 10.1007/s12264-021-00693-w
Oligodendroglial GABAergic Signaling: More Than Inhibition!
Abstract
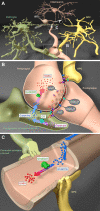
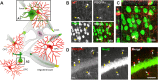
GABA is the main inhibitory neurotransmitter in the CNS acting at two distinct types of receptor: ligand-gated ionotropic GABAA receptors and G protein-coupled metabotropic GABAB receptors, thus mediating fast and slow inhibition of excitability at central synapses. GABAergic signal transmission has been intensively studied in neurons in contrast to oligodendrocytes and their precursors (OPCs), although the latter express both types of GABA receptor. Recent studies focusing on interneuron myelination and interneuron-OPC synapses have shed light on the importance of GABA signaling in the oligodendrocyte lineage. In this review, we start with a short summary on GABA itself and neuronal GABAergic signaling. Then, we elaborate on the physiological role of GABA receptors within the oligodendrocyte lineage and conclude with a description of these receptors as putative targets in treatments of CNS diseases.
Keywords: GABA; GABAA receptor; GABAB receptor; OPC; Oligodendrocyte lineage.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Lee SE, Lee Y, Lee GH. The regulation of glutamic acid decarboxylases in GABA neurotransmission in the brain. Arch Pharm Res. 2019;42:1031–1039. - PubMed
-
- Roberts E, Frankel S. gamma-Aminobutyric acid in brain: its formation from glutamic acid. J Biol Chem. 1950;187:55–63. - PubMed
-
- Krnjević K, Schwartz S. The action of gamma-aminobutyric acid on cortical neurones. Exp Brain Res. 1967;3:320–336. - PubMed
-
- Hösli L, Andrès PF, Hösli E. Neuron-glia interactions: indirect effect of GABA on cultured glial cells. Exp Brain Res. 1978;33:425–434. - PubMed
-
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

