Epigenetic transgenerational inheritance, gametogenesis and germline development†
- PMID: 33929020
- PMCID: PMC8444706
- DOI: 10.1093/biolre/ioab085
Epigenetic transgenerational inheritance, gametogenesis and germline development†
Abstract
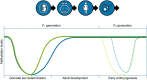
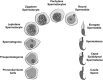
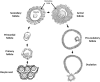
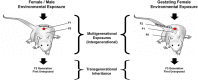
One of the most important developing cell types in any biological system is the gamete (sperm and egg). The transmission of phenotypes and optimally adapted physiology to subsequent generations is in large part controlled by gametogenesis. In contrast to genetics, the environment actively regulates epigenetics to impact the physiology and phenotype of cellular and biological systems. The integration of epigenetics and genetics is critical for all developmental biology systems at the cellular and organism level. The current review is focused on the role of epigenetics during gametogenesis for both the spermatogenesis system in the male and oogenesis system in the female. The developmental stages from the initial primordial germ cell through gametogenesis to the mature sperm and egg are presented. How environmental factors can influence the epigenetics of gametogenesis to impact the epigenetic transgenerational inheritance of phenotypic and physiological change in subsequent generations is reviewed.
Keywords: Epigenetics; Gametogenesis; Oogenesis; PGCs; Review; Spermatogenesis; Transgenerational.
© The Author(s) 2021. Published by Oxford University Press on behalf of Society for the Study of Reproduction.
Figures



References
-
- Tang WW, Kobayashi T, Irie N, Dietmann S, Surani MA. Specification and epigenetic programming of the human germ line. Nat Rev Genet 2016; 17:585–600. - PubMed
-
- Ohinata Y, Ohta H, Shigeta M, Yamanaka K, Wakayama T, Saitou M. A signaling principle for the specification of the germ cell lineage in mice. Cell 2009; 137:571–584. - PubMed
-
- Seydoux G, Dunn MA. Transcriptionally repressed germ cells lack a subpopulation of phosphorylated RNA polymerase II in early embryos of Caenorhabditis elegans and Drosophila melanogaster. Development 1997; 124:2191–2201. - PubMed
-
- Boswell RE, Mahowald AP. Tudor, a gene required for assembly of the germ plasm in Drosophila melanogaster. Cell 1985; 43:97–104. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

