Cataract-causing allele in CRYAA (Y118D) proceeds through endoplasmic reticulum stress in mouse model
- PMID: 33929105
- PMCID: PMC8175955
- DOI: 10.24272/j.issn.2095-8137.2020.354
Cataract-causing allele in CRYAA (Y118D) proceeds through endoplasmic reticulum stress in mouse model
Abstract
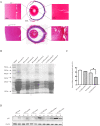
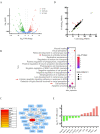
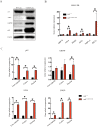
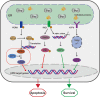
As small heat shock proteins, α-crystallins function as molecular chaperones and inhibit the misfolding and aggregation of β/γ-crystallins. Genetic mutations of CRYAA are associated with protein aggregation and cataract occurrence. One possible process underlying cataract formation is that endoplasmic reticulum stress (ERS) induces the unfolded protein response (UPR), leading to apoptosis. However, the pathogenic mechanism related to this remains unexplored. Here, we successfully constructed a cataract-causing CRYAA (Y118D) mutant mouse model, in which the lenses of the CRYAA-Y118D mutant mice showed severe posterior rupture, abnormal morphological changes, and aberrant arrangement of crystallin fibers. Histological analysis was consistent with the clinical pathological characteristics. We also explored the pathogenic factors involved in cataract development through transcriptome analysis. In addition, based on key pathway analysis, up-regulated genes in CRYAA-Y118D mutant mice were implicated in the ERS-UPR pathway. This study showed that prolonged activation of the UPR pathway and severe stress response can cause proteotoxic and ERS-induced cell death in CRYAA-Y118D mutant mice.
白内障是全球致盲率最高的眼科疾病,其发生发展与遗传、环境或衰老等造成的应激胁迫密切相关,发病机制较为复杂。目前,主流观点是大量未折叠蛋白累积,聚集沉淀,进而激活内质网应激(Endoplasmic Reticulum Stress, ERS),影响晶状体细胞命运。αA-晶体蛋白(CRYAA)作为小分子热休克蛋白,抑制β/γ-晶体蛋白错误折叠、聚集,维持晶体透明度发挥关键功能。研究报道,CRYAA遗传变异涉及先天性白内障或年龄相关性白内障,致病机制未阐述清楚。该研究成功构建人源遗传突变CRYAA-Y118D小鼠模型,突变型小鼠晶状体出现严重的后壁破裂、形态异常和晶体蛋白纤维排列异常等病理特征,与临床白内障病理特征一致。我们将通过转录组学探讨CRYAA-Y118D遗传突变致白内障的分子机制,差异基因的关键通路结果表明CRYAA-Y118D突变小鼠上调基因参与ERS-UPR通路。实验结果证实,CRYAA-Y118D分子伴侣功能缺陷导致UPR通路长期激活,剧烈应激反应导致聚集蛋白毒性和ERS诱导的细胞死亡。综上,该研究首次搭建人源遗传突变CRYAA-Y118D小鼠模型,揭示CRYAA-Y118D介导UPR-ERS影响晶状体细胞内蛋白质稳态及细胞命运决定,为白内障防治新策略提供动物模型和奠定理论依据。.
Keywords: Cataract; Endoplasmic reticulum stress; Unfolded protein response; αA-crystallin.
Figures

Similar articles
-
Trimethylamine N-oxide alleviates the severe aggregation and ER stress caused by G98R alphaA-crystallin.Mol Vis. 2009 Dec 19;15:2829-40. Mol Vis. 2009. PMID: 20029648 Free PMC article.
-
Probing the changes in gene expression due to α-crystallin mutations in mouse models of hereditary human cataract.PLoS One. 2018 Jan 16;13(1):e0190817. doi: 10.1371/journal.pone.0190817. eCollection 2018. PLoS One. 2018. PMID: 29338044 Free PMC article.
-
Mechanism of cataract formation in alphaA-crystallin Y118D mutation.Invest Ophthalmol Vis Sci. 2009 Jun;50(6):2919-26. doi: 10.1167/iovs.08-3070. Epub 2009 Jan 17. Invest Ophthalmol Vis Sci. 2009. PMID: 19151380 Free PMC article.
-
Genetics of crystallins: cataract and beyond.Exp Eye Res. 2009 Feb;88(2):173-89. doi: 10.1016/j.exer.2008.10.011. Epub 2008 Nov 1. Exp Eye Res. 2009. PMID: 19007775 Review.
-
sHSP in the eye lens: crystallin mutations, cataract and proteostasis.Int J Biochem Cell Biol. 2012 Oct;44(10):1687-97. doi: 10.1016/j.biocel.2012.02.015. Epub 2012 Mar 2. Int J Biochem Cell Biol. 2012. PMID: 22405853 Review.
Cited by
-
TRB3 Promotes Cataract Progression through Endoplasmic Reticulum Stress-mediated Mitochondrial Dysfunction and Cell Apoptosis.Cell Biochem Biophys. 2025 Mar;83(1):391-402. doi: 10.1007/s12013-024-01470-y. Epub 2024 Aug 8. Cell Biochem Biophys. 2025. PMID: 39115645
-
Insights on Human Small Heat Shock Proteins and Their Alterations in Diseases.Front Mol Biosci. 2022 Feb 25;9:842149. doi: 10.3389/fmolb.2022.842149. eCollection 2022. Front Mol Biosci. 2022. PMID: 35281256 Free PMC article. Review.
-
Engineered MEVs for photoreceptor-targeted delivery of USP25 to alleviate diabetic retinopathy.J Nanobiotechnology. 2025 Aug 20;23(1):575. doi: 10.1186/s12951-025-03671-w. J Nanobiotechnology. 2025. PMID: 40830790 Free PMC article.
-
Expression of αA-crystallin (CRYAA) in vivo and in vitro models of age-related cataract and the effect of its silencing on HLEB3 cells.Aging (Albany NY). 2023 May 28;15(10):4498-4509. doi: 10.18632/aging.204754. Epub 2023 May 28. Aging (Albany NY). 2023. PMID: 37253645 Free PMC article.
-
Identification of mutations associated with congenital cataracts in nineteen Chinese families.BMC Ophthalmol. 2025 Feb 25;25(1):94. doi: 10.1186/s12886-025-03920-4. BMC Ophthalmol. 2025. PMID: 39994538 Free PMC article.
References
-
- Al Mamun A, Rahman MM, Zaman S, Munira MS, Uddin MS, Rauf A, et al Molecular Insight into the Crosstalk of UPS Components and Alzheimer’s Disease. Current Protein & Peptide Science. 2020;21(12):1193–1201. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
