SARS-CoV-2 Antibodies in Adult Patients With Multiple Sclerosis in the Amsterdam MS Cohort
- PMID: 33929488
- PMCID: PMC8087977
- DOI: 10.1001/jamaneurol.2021.1364
SARS-CoV-2 Antibodies in Adult Patients With Multiple Sclerosis in the Amsterdam MS Cohort
Abstract
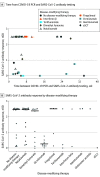
This cohort study assesses a group of patients with multiple sclerosis in Amsterdam, the Netherlands, for SARS-CoV-2 antibodies to quantify asymptomatic infections and immunological response to COVID-19.
Conflict of interest statement
Figures
Comment in
-
SARS-CoV-2 antibodies in multiple sclerosis patients depending on the vaccine mode of action?Mult Scler. 2022 Jan;28(1):165-167. doi: 10.1177/13524585211039128. Epub 2021 Aug 13. Mult Scler. 2022. PMID: 34387536 No abstract available.
References
-
- Vogelzang EH, Loeff FC, Derksen NIL, et al. ; Amsterdam University Medical Center COVID-19 Biobank Study Group . Development of a SARS-CoV-2 total antibody assay and the dynamics of antibody response over time in hospitalized and nonhospitalized patients with COVID-19. J Immunol. 2020;205(12):3491-3499. doi:10.4049/jimmunol.2000767 - DOI - PubMed
-
- Zabalza A, Cárdenas-Robledo S, Tagliani P, et al. . COVID-19 in multiple sclerosis patients: susceptibility, severity risk factors and serological response. Eur J Neurol. 2020. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

