Effects of Hospital-Based Comprehensive Medication Reviews Including Postdischarge Follow-up on Older Patients' Use of Health Care: A Cluster Randomized Clinical Trial
- PMID: 33929523
- PMCID: PMC8087955
- DOI: 10.1001/jamanetworkopen.2021.6303
Effects of Hospital-Based Comprehensive Medication Reviews Including Postdischarge Follow-up on Older Patients' Use of Health Care: A Cluster Randomized Clinical Trial
Erratum in
-
Incorrect Trial Registration Identifier.JAMA Netw Open. 2022 Apr 1;5(4):e229745. doi: 10.1001/jamanetworkopen.2022.9745. JAMA Netw Open. 2022. PMID: 35380650 Free PMC article. No abstract available.
Abstract
Importance: Suboptimal use of medications is a leading cause of health care-related harm. Medication reviews improve medication use, but evidence of the possible benefit of inpatient medication review for hard clinical outcomes after discharge is scarce.
Objective: To study the effects of hospital-based comprehensive medication reviews (CMRs), including postdischarge follow-up of older patients' use of health care resources, compared with only hospital-based reviews and usual care.
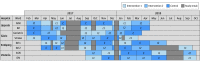
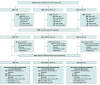
Design, setting, and participants: The Medication Reviews Bridging Healthcare trial is a cluster randomized crossover trial that was conducted in 8 wards with multiprofessional teams at 4 hospitals in Sweden from February 6, 2017, to October 19, 2018, with 12 months of follow-up completed December 6, 2019. The study was prespecified in the trial protocol. Outcome assessors were blinded to treatment allocation. In total, 2644 patients aged 65 years or older who had been admitted to 1 of the study wards for at least 1 day were included. Data from the modified intention-to-treat population were analyzed from December 10, 2019, to September 9, 2020.
Interventions: Each ward participated in the trial for 6 consecutive 8-week periods. The wards were randomized to provide 1 of 3 treatments during each period: CMR, CMR plus postdischarge follow-up, and usual care without a clinical pharmacist.
Main outcomes and measures: The primary outcome measure was the incidence of unplanned hospital visits (admissions plus emergency department visits) within 12 months. Secondary outcomes included medication-related admissions, visits with primary care clinicians, time to first unplanned hospital visit, mortality, and costs of hospital-based care.
Results: Of the 2644 participants, 7 withdrew after inclusion, leaving 2637 for analysis (1357 female [51.5%]; median age, 81 [interquartile range, 74-87] years; median number of medications, 9 [interquartile range, 5-13]). In the modified intention-to-treat analysis, 922 patients received CMR, 823 received CMR plus postdischarge follow-up, and 892 received usual care. The crude incidence rate of unplanned hospital visits was 1.77 per patient-year in the total study population. The primary outcome did not differ between the intervention groups and usual care (adjusted rate ratio, 1.04 [95% CI, 0.89-1.22] for CMR and 1.15 [95% CI, 0.98-1.34] for CMR plus postdischarge follow-up). However, CMR plus postdischarge follow-up was associated with an increased incidence of emergency department visits within 12 months (adjusted rate ratio, 1.29; 95% CI, 1.05-1.59) compared with usual care. There were no differences between treatment groups regarding other secondary outcomes.
Conclusions and relevance: In this study of older hospitalized patients, CMR plus postdischarge follow-up did not decrease the incidence of unplanned hospital visits. The findings do not support the performance of hospital-based CMRs as conducted in this trial. Alternative forms of medication reviews that aim to improve older patients' health outcomes should be considered and subjected to randomized clinical trials.
Trial registration: ClinicalTrials.gov Identifier: NCT02986425.
Conflict of interest statement
Figures
Comment in
-
Optimization of Medication by Pharmacists in Older People With Multimorbidity for Improved Outcomes-Mirage or Reality?JAMA Netw Open. 2021 Apr 1;4(4):e216392. doi: 10.1001/jamanetworkopen.2021.6392. JAMA Netw Open. 2021. PMID: 33929527 No abstract available.
References
-
- Aitken M, Gorokhovich L. Advancing the Responsible Use of Medicines: Applying Levers for Change. IMS Institute for Healthcare Informatics; 2012. doi:10.2139/ssrn.2222541 - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

