Efficacy and Safety of Budesonide/Glycopyrronium/Formoterol Fumarate versus Other Triple Combinations in COPD: A Systematic Literature Review and Network Meta-analysis
- PMID: 33929661
- PMCID: PMC8189959
- DOI: 10.1007/s12325-021-01703-z
Efficacy and Safety of Budesonide/Glycopyrronium/Formoterol Fumarate versus Other Triple Combinations in COPD: A Systematic Literature Review and Network Meta-analysis
Abstract
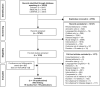
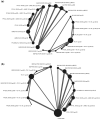
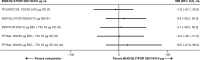
In patients with chronic obstructive pulmonary disease (COPD) who experience further exacerbations or symptoms, despite being prescribed dual long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA) or inhaled corticosteroid (ICS)/LABA therapies, triple ICS/LAMA/LABA therapy is recommended. A previous network meta-analysis showed comparable efficacy of the ICS/LAMA/LABA, budesonide/glycopyrronium bromide/formoterol fumarate (BUD/GLY/FOR) 320/18/9.6 µg, to other fixed-dose and open combination triple therapies at 24 weeks in COPD. Subsequently, the ETHOS study was published, including data for 8509 patients, assessing the efficacy and safety of BUD/GLY/FOR over 52 weeks. This network meta-analysis (NMA) was conducted to compare the relative efficacy, safety, and tolerability of BUD/GLY/FOR 320/18/9.6 µg with other fixed-dose and open combination triple therapies in COPD over 52 weeks, including data from ETHOS. A systematic literature review was conducted to identify ≥ 10-week randomized controlled trials, including ≥ 1 fixed-dose or open combination triple-therapy arm, in patients with moderate-to-very severe COPD. The methodologic quality and risk of bias of included studies were assessed. Study results were combined using a three-level hierarchical Bayesian NMA model to assess efficacy and safety outcomes at or over 24 and 52 weeks. Meta-regression and sensitivity analyses were used to assess heterogeneity across studies. Nineteen studies (n = 37,741 patients) met the inclusion criteria of the review; 15 contributed to the base case network. LAMA/LABA dual combinations were combined as a single treatment group to create a connected network. Across all outcomes for exacerbations, lung function, symptoms, health-related quality of life, safety, and tolerability, the efficacy and safety of BUD/GLY/FOR were comparable to those of other triple ICS/LAMA/LABA fixed-dose (fluticasone furoate/umeclidinium/vilanterol and beclomethasone dipropionate/glycopyrronium bromide/formoterol fumarate) and open combinations at or over 24 and 52 weeks. Sensitivity analyses and meta-regression results for exacerbation outcomes were broadly in line with the base case NMA. In this NMA, BUD/GLY/FOR 320/18/9.6 μg showed comparable efficacy versus other ICS/LAMA/LABA fixed-dose or open combination therapies in terms of reducing exacerbation rates and improving lung function, symptoms and health-related quality of life in patients with moderate-to-very-severe COPD, in line with previously published meta-analysis results of triple combinations in COPD. The safety and tolerability profile of BUD/GLY/FOR was also found to be comparable to other triple combination therapies.
Keywords: Chronic obstructive pulmonary disease; Exacerbations; Inhaled corticosteroid; Long-acting muscarinic antagonist; Long-acting β2-agonist; Lung function; Network meta-analysis; Patient-reported outcomes; Safety; Triple therapy.
Figures




References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2021 report. 2021. https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.0-16.... Accessed 18 Nov 2020.
-
- Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388:963–973. doi: 10.1016/S0140-6736(16)31354-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

