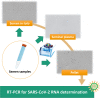
Molecular diagnosis of SARS-CoV-2 in seminal fluid
- PMID: 33929709
- PMCID: PMC8085093
- DOI: 10.1007/s40618-021-01580-x
Molecular diagnosis of SARS-CoV-2 in seminal fluid
Abstract
Purpose: Due to relevant repercussions on reproductive medicine, we aimed to evaluate feasibility of RT-PCR as a detection method of SARS-CoV-2 RNA in seminal fluid.
Methods: A qualitative determination of the RT-PCR assays in semen was performed through different approaches: (1) efficiency of RNA extraction from sperm and seminal plasma was determined using PRM1 and PRM2 mRNA and a heterologous system as control; (2) samples obtained by diluting viral preparation from a SARS-CoV-2 panel (virus cultured in Vero E6 cell lines) were tested; (3) viral presence in different fractions of seminal fluid (whole sample, seminal plasma and post-centrifugation pellet) was evaluated. Semen samples from mild and recovered COVID-19 subjects were collected by patients referring to the Infectious Disease Department of the Policlinico Umberto I Hospital - "Sapienza" University of Rome. Control subjects were recruited at the Laboratory of Seminology-Sperm Bank "Loredana Gandini'' of the same hospital.
Results: The control panel using viral preparations diluted in saline and seminal fluid showed the capability to detect viral RNA presence with Ct values depending on the initial viral concentration. All tested semen samples were negative for SARS-CoV-2, regardless of the nasopharyngeal swab result or seminal fluid fraction.
Conclusion: These preliminary data show that RT-PCR for SARS-CoV-2 RNA testing appears to be a feasible method for the molecular diagnosis of SARS-CoV-2 in seminal fluid, supported by results of the control panel. The ability to detect SARS-CoV-2 in semen is extremely important for reproductive medicine, especially in assisted reproductive technology and sperm cryopreservation.
Keywords: COVID-19; RT-PCR; SARS-CoV-2; Seminal fluid.
© 2021. The Author(s).
Conflict of interest statement
All the authors declare that they have no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous