TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors
- PMID: 33929895
- PMCID: PMC8315301
- DOI: 10.1200/JCO.20.03489
TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors
Abstract
Purpose: Patients with metastatic urothelial carcinoma (mUC) who progress on platinum-based combination chemotherapy (PLT) and checkpoint inhibitors (CPIs) have limited options that offer objective response rates (ORRs) of approximately 10% with a median overall survival (OS) of 7-8 months. Sacituzumab govitecan (SG) is a TROP-2-directed antibody-drug conjugate with an SN-38 payload that has shown preliminary activity in mUC.
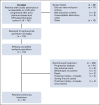
Methods: TROPHY-U-01 (ClinicalTrials.gov identifier: NCT03547973) is a multicohort, open-label, phase II, registrational study. Cohort 1 includes patients with locally advanced or unresectable or mUC who had progressed after prior PLT and CPI. Patients received SG 10 mg/kg on days 1 and 8 of 21-day cycles. The primary outcome was centrally reviewed ORR; secondary outcomes were progression-free survival, OS, duration of response, and safety.
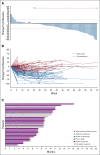
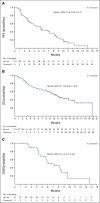
Results: Cohort 1 included 113 patients (78% men; median age, 66 years; 66.4% visceral metastases; median of three [range, 1-8] prior therapies). At a median follow-up of 9.1 months, the ORR was 27% (31 of 113; 95% CI, 19.5 to 36.6); 77% had decrease in measurable disease. Median duration of response was 7.2 months (95% CI, 4.7 to 8.6 months), with median progression-free survival and OS of 5.4 months (95% CI, 3.5 to 7.2 months) and 10.9 months (95% CI, 9.0 to 13.8 months), respectively. Key grade ≥ 3 treatment-related adverse events included neutropenia (35%), leukopenia (18%), anemia (14%), diarrhea (10%), and febrile neutropenia (10%), with 6% discontinuing treatment because of treatment-related adverse events.
Conclusion: SG is an active drug with a manageable safety profile with most common toxicities of neutropenia and diarrhea. SG has notable efficacy compared with historical controls in pretreated mUC that has progressed on both prior PLT regimens and CPI. The results from this study supported accelerated approval of SG in this population.
Conflict of interest statement
Figures

Comment in
-
Sacituzumab govitecan is safe and effective.Nat Rev Clin Oncol. 2021 Jul;18(7):400. doi: 10.1038/s41571-021-00523-y. Nat Rev Clin Oncol. 2021. PMID: 33986521 No abstract available.
-
ADC sacituzumab govitecan shows efficacy and safety.Nat Rev Urol. 2021 Jul;18(7):384. doi: 10.1038/s41585-021-00479-9. Nat Rev Urol. 2021. PMID: 34002071 No abstract available.
-
Beyond Chemotherapy and Checkpoint Inhibitors: Weighing the Risks and Benefits of the Novel Therapies for Metastatic Urothelial Carcinoma.J Clin Oncol. 2021 Oct 20;39(30):3411-3412. doi: 10.1200/JCO.21.01430. Epub 2021 Sep 7. J Clin Oncol. 2021. PMID: 34491780 No abstract available.
-
Reply to T. Powles et al.J Clin Oncol. 2021 Oct 20;39(30):3412-3413. doi: 10.1200/JCO.21.01673. Epub 2021 Sep 7. J Clin Oncol. 2021. PMID: 34491818 No abstract available.
References
-
- National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology: Bladder Cancer Version 5.2020. https://www.nccn.org/professionals/physician_gls/
-
- Petrylak DP de Wit R Chi KN, et al. : Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): A randomised, double-blind, phase 3 trial. Lancet 390:2266-2277, 2017 - PubMed
-
- Raggi D Miceli R Sonpavde G, et al. : Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: A systematic review and meta-analysis. Ann Oncol 27:49-61, 2016 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

