A pilot study of oral treprostinil pharmacogenomics and treatment persistence in patients with pulmonary arterial hypertension
- PMID: 33929912
- PMCID: PMC8111525
- DOI: 10.1177/17534666211013688
A pilot study of oral treprostinil pharmacogenomics and treatment persistence in patients with pulmonary arterial hypertension
Abstract
Background and aims: Treprostinil is a prostacyclin analog used to treat pulmonary arterial hypertension. Dosing is empiric and based on tolerability. Adverse effects are common and can affect treatment persistence. Pharmacogenomic variants that may affect treprostinil metabolism and transport have not been well-characterized. We aimed to investigate the pharmacogenomic sources of variability in treatment persistence and dosing.
Methods: Patients were prospectively recruited from an IRB approved biobank registry at a single pulmonary hypertension center. A cohort of patients who received oral treprostinil were screened for participation. Pharmacogenomic analysis was for variants in CYP2C8, CYP2C9, and ABCC4. A retrospective review was conducted for demographics, clinical status, dosing, and response. Fisher's exact test was used for categorical data and Kruskal-Wallis test or Wilcoxon rank sum were used for continuous data.
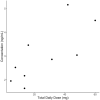
Results: A total of 15 patients received oral treprostinil and were consented. Their median age was 53 years, 73% were female, and 93% were White. The median total daily dose was 22.5 mg (13.5, 41) at last clinical observation. 40% of patients discontinued treatment with a majority due to adverse effects. Approximately 27% of patients had a loss-of-function variant in CYP2C8 (*1/*3 or *1/*4), whereas 47% of patients had a loss-of-function variant in CYP2C9 (*1/*2, *1/*3, or *2/*2). Minor allele frequencies for ABCC4 (rs1751034 and rs3742106) were 0.17 and 0.43, respectively. Survival analysis showed that increased CYP2C9 activity score was associated with decreased risk for treatment discontinuation [hazard ratio (HR): 0.13; 95% confidence interval (CI): 0.02, 0.91; p = 0.04]. Genetic variants were not significantly associated with dosing.
Conclusion: Genetic variants responsible for the metabolism and transport of oral treprostinil were common. Increased CYP2C9 activity score was associated with decreased risk for treatment discontinuation. However, dosing was not associated with genetic variants in metabolizing enzymes for treprostinil. Our findings suggest significant variability in treatment persistence to oral treprostinil, with pharmacogenomics being a potentially important contributor.The reviews of this paper are available via the supplemental material section.
Keywords: personalized medicine; pharmacogenomics; pulmonary arterial hypertension; treprostinil.
Conflict of interest statement
Karryn Crisamore has no relevant disclosures.
Solomon Adams has no relevant disclosures.
Ashley Modany has no relevant disclosures.
Marc A. Simon has received research support from Novartis and Aadi, and has received consulting fees from United Therapeutics, Actelion, Complexa, Gilead, Acceleron.
Wenchen Zhao has no relevant disclosures.
Imam H Shaik has no relevant disclosures.
Raman Venkataramanan has received grant funding from United Therapeutics.
Philip Empey has no relevant disclosures.
Figures




Similar articles
-
Pharmacogenomics in the Management of Pulmonary Arterial Hypertension: Current Perspectives.Pharmgenomics Pers Med. 2023 Jul 11;16:729-737. doi: 10.2147/PGPM.S361222. eCollection 2023. Pharmgenomics Pers Med. 2023. PMID: 37457231 Free PMC article. Review.
-
Safety and tolerability of transition from inhaled treprostinil to oral selexipag in pulmonary arterial hypertension: Results from the TRANSIT-1 study.J Heart Lung Transplant. 2019 Jan;38(1):43-50. doi: 10.1016/j.healun.2018.09.003. Epub 2018 Sep 12. J Heart Lung Transplant. 2019. PMID: 30391194 Clinical Trial.
-
Practical management of oral treprostinil in patients with pulmonary arterial hypertension: Lessons from ADAPT, EXPEDITE, and expert consensus.Respir Med. 2024 Sep;231:107734. doi: 10.1016/j.rmed.2024.107734. Epub 2024 Jul 8. Respir Med. 2024. PMID: 38986791
-
Inpatient Initiation of Oral Treprostinil in an Academic Medical System.Cardiovasc Drugs Ther. 2020 Aug;34(4):547-553. doi: 10.1007/s10557-020-06992-0. Cardiovasc Drugs Ther. 2020. PMID: 32424651
-
Pharmacokinetic evaluation of treprostinil (oral) for the treatment of pulmonary arterial hypertension.Expert Opin Drug Metab Toxicol. 2014 Oct;10(10):1445-53. doi: 10.1517/17425255.2014.958466. Epub 2014 Sep 10. Expert Opin Drug Metab Toxicol. 2014. PMID: 25204984 Review.
Cited by
-
Simulation of clinical trials of oral treprostinil in pulmonary arterial hypertension using a virtual population.NPJ Syst Biol Appl. 2025 Jan 15;11(1):9. doi: 10.1038/s41540-024-00481-y. NPJ Syst Biol Appl. 2025. PMID: 39814773 Free PMC article.
-
Pharmacogenomics in the Management of Pulmonary Arterial Hypertension: Current Perspectives.Pharmgenomics Pers Med. 2023 Jul 11;16:729-737. doi: 10.2147/PGPM.S361222. eCollection 2023. Pharmgenomics Pers Med. 2023. PMID: 37457231 Free PMC article. Review.
-
Differential drug response in pulmonary arterial hypertension: The potential for precision medicine.Pulm Circ. 2023 Nov 2;13(4):e12304. doi: 10.1002/pul2.12304. eCollection 2023 Oct. Pulm Circ. 2023. PMID: 37927610 Free PMC article. Review.
References
-
- Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 2004; 351: 1425–1436. - PubMed
-
- Gomberg-Maitland M, Glassner-Kolmin C, Watson S, et al. Survival in pulmonary arterial hypertension patients awaiting lung transplantation. J Heart Lung Transplant 2013; 32: 1179–1186. - PubMed
-
- Coons JC, Pogue K, Kolodziej AR, et al. Pulmonary arterial hypertension: a pharmacotherapeutic update. Curr Cardiol Rep 2019; 21: 141. - PubMed
-
- United Therapeutics Corporation. Orenitram (treprostinil) [package insert]. Research Triangle Park, NC: United Therapeutics Corporation, 2017.

